NAFDAC alert on suspected fake Augmentin tablets and wetin you suppose know
Wia dis foto come from, NAFDAC
Nigeria joinbodi for Food and Drug Administration and Control don draw di attention of di public say dem don detect one suspected fake Augmentin 625mg tablets wey dey circulate within di di kontri.
Na dis reason make NAFDAC draw ear give all dia formations for di zones and 36 states of di federation including di FCT say make dem carry out surveillance and mop up di falsified Augmentin tablets.
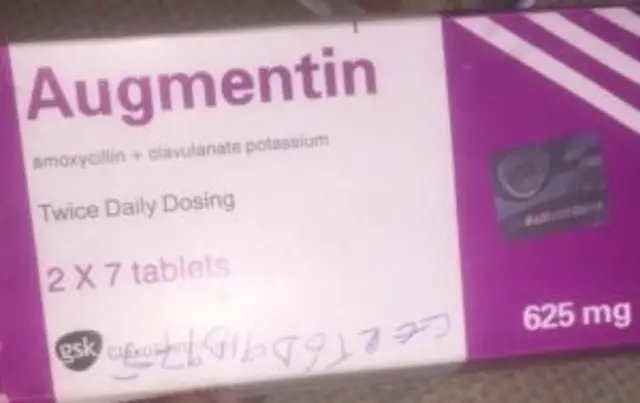
According to NAFDAC, di product no meet labeling requirements.
Dem add say e no get di name of di manufacturer only di address dey dia.
Manufacturing and Expiry dates no also meet di acceptable format.
E no get MAS scratch number wey pesin go take verify say na original.
Di logo “gsk” no dey properly positioned as e be for di original.
Di agency add say ”di listed informate show say di product be fake and counterfeit.
End of Di one wey oda users dey read well well

Wia dis foto come from, NAFDAC

Wia dis foto come from, NAFDAC
Wetin to look out for di fake Augmentin 625mg Tabs
- Product Name: Augmentin 625mg.
- Batch No.: 562626
- Manufacturing date: April 2021
- Expiry date: April 2024.
- NAFDAC Reg No: 04-1928.
- Labelling errors
How to sabi original Augmentin
NAFDAC say make pipo take note say di original Augmentin 625mg get:
- Better product label informate
- Date markings
- Expiration and Manufacture dates
- Batch number and
- NAFDAC registration number.
All those be something wey pesin go fit read.
NAFDAC also draw ear give wholesalers, distributors, and pharmacies say make dem dey buy medicines from authorized/licensed suppliers’
And make dem increase as dem dey chook eye inside di mata within di supply chain so dem go fit avoid make fake product dey enta market.
De say make dem also make sure say products authenticity, physical condition and labels na sometin wey dem carefully checked.
NAFDAC also advise healthcare providers make dem dey vigilant so dem no go give fake products to unsuspecting patient.
Also make members of di public wey get di suspected counterfeit product stop to sell or use am, and submit di stock to di nearest NAFDAC office.
Oda times wey di health joinbodi recall drugs from di market
Dexamethasone
Early dis month, di National Agency for Food and Drugs Administration and Control (NAFDAC) bin recall substandard dexamethasone tablets wey dem see for Anambra State.
Dem recall am sake of unsatisfactory results of analysis wey show say di samples contain less dan di amount wey dey for di labels.
Some of di samples also contain small amounts of methylparaben and/or propylparaben.
Methylparaben and/or propylparaben na preservative wey pipo dey use to make product get longer shelf life.
NAFDAC tok say di presence of dis preservatives inside tablets no dey normal and e dey unnecessary.
Dem draw ear for kontri pipo to avoid dis substances especially for paediatric (children) formulations.

Wia dis foto come from, @Nafdac
Cough Syrup mata
For di same October, NAFDAC bin chook mouth for di four children cold and cough Syrup mata wey di World Health Organization (WHO) dey draw pipo ear about around di world.
Oga of NADFDAC Prof. Mojisola Adeyeye for Press Conference for Abuja say "WHO say di four cold and cough syrups dey linked to serious kidney injuries and 66 deaths among pikin dem for The Gambia".
Dem add say di syrups cause serious kidney injuries and di death of children for July, August, and September for The Gambia.
NAFDAC say dem dey make sure say dis products no cause harm to Nigerians.
Dem don also give alert to di public on di Substandard (contaminated) children cough syrups wey dey spread for Gambia.
"We don put appropriate measures in place to make sure say dis product no enta our kontri from various ports of entry, we don also activate our internal surveillance mechanisms to mop up dis products from di supply chain pipeline if dem ever find way enta hia.
Di Cough syrups na Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup wey one Indian company manufacture.














