What are the key learning points about electrochemistry?
Electrolysis is the decomposedIf a substance decomposes, it breaks down into simpler compounds or elements. of a liquid electrolyteA liquid or solution which is a conductor of electricity and is decomposed (broken down) by it. Rainwater, tap water and salt water are all examples of electrolytes. using a direct currentDirect current is the movement of charge through a conductor in one direction only. of electricity. Separated elements form at two electrodeA conductor used to establish electrical contact with a circuit. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The electrode attached to the positive terminal of a battery is the positive electrode, or anode.: the negative cathode and the positive anode.
Molten lead(II) bromide can be broken down into lead metal and bromine gas, and lithium chloride can be broken down into lithium metal and chlorine gas. Oxygen and hydrogen gases can be obtained from electrolysis of dilute sulfuric acid.
Aluminium metal can be extracted from its oreA rock that contains enough of a metal or a metal compound to make extracting it economically worthwhile.bauxite, in a large-scale industrial electrolysis process.
What is electrolysis?
ionic compoundAn ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom that has lost an electron). are made up of oppositely charged ionAn atom or molecule that has either a positive or negative electrical charge..
Electrical energy can be used to split an ionic compound into separate elements.
For example, lithium bromide can be split up into lithium and bromine.
An electrolyte is a liquid or solution which conducts electricity and is decomposed by it.
Electrolysis is the decomposition of a liquid electrolyte using a direct current of electricity.
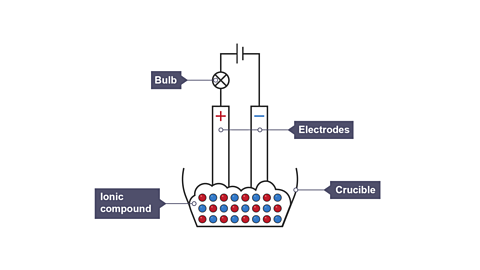
Image caption, 1. Two electrodes are attached to a direct current and inserted into an ionic compound. The bulb does not light as the ions are held in fixed positions so no current can flow.
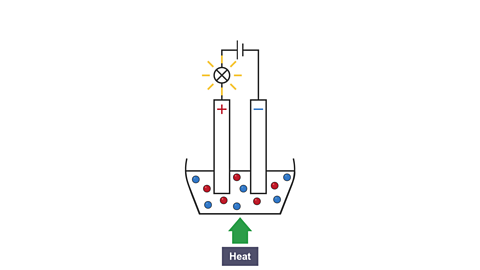
Image caption, 2. The crucible is heated and the solid melts. The bulb lights as the ions are free to move and carry charge.
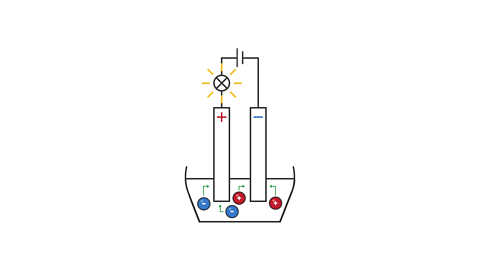
Image caption, 3. The positive ions (cations) are attracted to the negative electrode (cathode), and the negative ions (anions) are attracted to the positive electrode (anode).
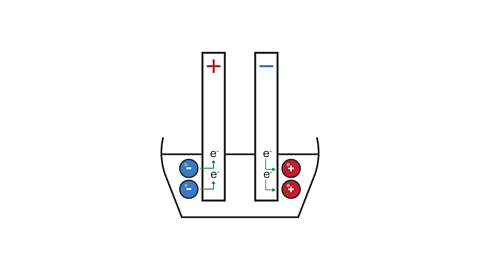
Image caption, 4. The anode takes electrons away from the anions, and the cathode gives electrons to the cations. This allows the compound to be split into separate neutral elements.
1 of 4
What are electrodes?
electrodeA conductor used to establish electrical contact with a circuit. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The electrode attached to the positive terminal of a battery is the positive electrode, or anode. are inserted into the chemical that is being broken down in the electrolysis reaction.
The positively charged electrode is called the anode.
- Negatively charged ions (anions) move towards the anode where they lose electronSubatomic particle, with a negative charge and a very small mass relative to protons and neutrons..
The negatively charged electrode is called the cathode.
- Positively charged ions (cations) move towards the cathode where they gain electrons.
Graphite electrodes are inert electrodes because they do not take part in the electrolysis reactions.
Instead, they provide a surface on which these reactions can happen.
How is electrolysis used with ionic compounds?
ionic compoundAn ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom that has lost an electron). can be broken down into separate elements using electrolysis.
Two examples of this are in lead(II) bromide and lithium chloride.
What happens during the electrolysis of lead(II) bromide (PbBr₂)?
The following apparatus is used:
Lead(II) bromide (PbBr2) is made up of lead(II) ionAn atom or molecule that has either a positive or negative electrical charge. (Pb2+) and bromide ions (Br-).
Once the solid compound is melted, current begins to flow and the ions are attracted to the electrodes.
What is the reaction at cathode (negative electrode)?
The negative cathode attracts the positive Pb2+ cations which gain electrons to turn into liquid lead metal (Pb).
Observations: silvery grey liquid (sinks to bottom of crucible).
Half equation: Pb2+ + 2e- → Pb (higher tier only).
What is the reaction at the anode (positive electrode)?
The positive anode attracts the negative Br- anions which lose electrons to turn into gaseous bromine (Br2).
Observations: red-brown pungent gas.
Half equation: 2Br- → Br2 + 2e- (higher tier only).
What happens during the electrolysis of lithium chloride (LiCl)?
The same apparatus is used in the electrolysis of lithium chloride that was used for lead(II) bromide.
Lithium chloride (LiCl) is made up of lithium ions (Li+) and chloride ions (Cl-).
Once the solid compound is melted, current begins to flow and the ions are attracted to the electrodes.
What is the reaction at the cathode (negative electrode)?
The negative cathode attracts the positive Li+ cations which gain electrons to turn into liquid lithium metal (Li).
Observations: silvery grey liquid observed around electrode.
Half equation: Li+ + e- → Li (higher tier only).
What is the reaction at the anode (positive electrode)?
The positive anode attracts the negative Cl- anions which lose electrons to turn into gaseous chlorine (Cl2).
Observations: yellow-green pungent gas.
Half equation: 2Cl- → Cl2 + 2e- (higher tier only).
How to predict the electrolysis of an ionic compound
In the electrolysisThe decomposition (breaking down) of a liquid electrolyte using a direct current of electricity. Separated elements form at two electrodes: the negative cathode and the positive anode. of any ionic compoundAn ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom that has lost an electron)., the metal is observed at the negative cathode, and the non-metal is observed at the positive anode.
The observations for the formation of a metal in electrolysis is usually ‘grey liquid’ (apart from copper which is red-brown liquid).
The observations for the formation of a non-metal in electrolysis depends on the colour of the gas:
| Non-metal | Observation in electrolysis experiment |
|---|---|
| Oxygen (O2), Nitrogen (N2), Hydrogen (H2) | Colourless gas |
| Fluorine (F2) | Yellow gas |
| Chlorine (Cl2) | Yellow-green gas |
| Bromine (Br2) | Red-brown gas |
| Iodine (I2) | Purple gas |
Key fact
Non-metals normally appear as gases in electrolysis experiments even if they are liquids at room temperature.
This is due to the high temperatures involved in electrolysis.
Question
An electrolysis experiment is carried out on calcium iodide (CaI2).
Predict the observations at the anode and the cathode.
(Higher tier only) Write half equations for the reactions that take place.
Answer
Calcium Iodide is made up of calcium ions (Ca2+) and iodide ions (I-).
Calcium metal will be formed at the cathode, and iodine gas will be formed at the anode.
Cathode
Observation: grey liquid (calcium)
Half equation: Ca2+ + 2e- → Ca (higher tier only)
Anode
Observation: purple gas (iodine)
Half equation: 2I- → I2 + 2e- (higher tier only)
What happens during the electrolysis of dilute sulfuric acid?
Dilute sulfuric acid is a mixture made up of sulfuric acid (H2SO4) and water (H2O).
The ions present in this mixture are H+ and OH- (from the water) and H+ and SO42- from the sulfuric acid.
What is the reaction at the cathode?
The H+ ions are attracted to the negative cathode, gain electrons and form hydrogen gas.
Observation: colourless gas.
Half equation: 2H+ + 2e- → H2 (higher tier only)
What is the reaction at the anode?
Both OH- and SO42- ions are attracted to the positive anode.
However, it is only the hydroxide ions that react by losing electrons to form oxygen gas.
Observation: colourless gas.
Half equation: 4OH- → O2 + 2H2O + 4e- (higher tier only)
The volume of hydrogen produced at the cathode is twice the volume of oxygen produced at the anode.
How is aluminium extracted?
Aluminium is a reactive metal, so it cannot be found unreacted in the Earth.
Electrolysis is used to extract aluminium from the oreA rock that contains enough of a metal or a metal compound to make extracting it economically worthwhile.bauxite which contains aluminium oxide (Al2O3).
What electrolyte is used to obtain aluminium and oxygen?
Bauxite is processed to produce alumina, which is pure aluminium oxide.
This is the electrolyte which is broken down using electrolysis to obtain aluminium and oxygen.
However, the melting point of aluminium oxide is very high (over 2000oC).
Aluminium oxide is therefore dissolved in molten cryolite.
This mixture melts at a lower temperature than aluminium oxide (900-1000oC) which reduces energy costs.
Cryolite also increases conductivity.
During the electrolysis an aluminium oxide crust forms on top of the molten cryolite which helps to trap heat.
What is the electrolysis process for extracting aluminium?
The diagram shows an electrolysis cell used to extract aluminium.
The negative cathode forms the sides and base of the cell, and the positive anodes are inserted at the top of the cell.
What is the reaction at the cathode?
The aluminium ions (Al3+) are attracted to the negative cathode, gain electrons and form liquid aluminium.
The liquid aluminium sinks to the bottom of the cell and is tapped off.
Half equation: Al3+ + 3e- → Al (Higher tier only).
What is the reaction at the anode?
The oxide ions (O2-) are attracted to the positive anode, lose electrons and form oxygen gas.
Half equation: 2O2- → O2 + 4e- (Higher tier only).
The oxygen reacts with the carbon anodes, forming carbon dioxide (C + O2 → CO2).
This causes the anodes to gradually wear away.
They must be replaced frequently, adding to the cost of producing aluminium.
Why is aluminium recycled?
Extracting aluminium from its ore is expensive because its electrolysis requires a lot of energy.
Recycling aluminium uses far less energy and is therefore much cheaper than extracting fresh aluminium from bauxite.
How much do you know about electrochemistry?
More on Unit 2: Further chemical reactions, rates and equilibrium, calculations and organic chemistry
Find out more by working through a topic
- count8 of 9
- count9 of 9

- count1 of 9

- count2 of 9
