Unit 2: Further chemical reactions, rates and equilibrium, calculations and organic chemistry
Metals and the reactivity series
Learn about how the reactivity series ranks metals by how readily they react. More reactive metals displace less reactive metals from their compounds and react with water.

Redox, rusting and iron
Learn about how oxidation is loss of electrons, gain of oxygen or loss of hydrogen. Reduction is gain of electrons, loss of oxygen or gain of hydrogen.

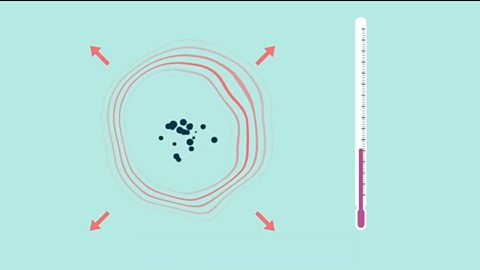
Rates of reaction
Learn how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface area or by using a catalyst.

Equilibrium
Learn and revise the Haber process, Le Chatelier's principle, reversible reactions, the symbol for this and dynamic equilibrium.

Organic chemistry
Learn about how an organic chemical contains the element carbon and the four different homologous series of organic compounds: alkanes, alkenes, alcohols and carboxylic acids.

Quantitative chemistry (2)
Learn how to use moles of a solute dissolved in a solution and moles of a gas to carry out calculations.

Electrochemistry
Learn about electrochemistry and explore the principles behind electrolysis, oxidation and reduction equations and extracting aluminium.

Energy changes in chemistry
Learn about chemical reactions and how energy is transferred to or from the surroundings. Find out about reactions and temperature changes and ways of calculating energy changes.
Gas chemistry
Learn about the gaseous elements and compounds in the Earth’s atmosphere; nitrogen, ammonia, hydrogen, oxygen, metal and non-metal oxides and carbon dioxide.

Links
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link