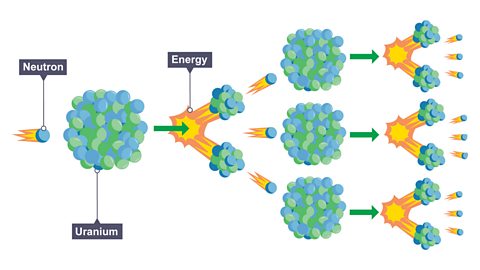
What are the key learning points about the structure of the atom?
atomAll elements are made of atoms. An atom consists of a nucleus containing protons and neutrons, surrounded by electrons. are overall electrically neutral.
Atoms contain positive protonSubatomic particle with a positive charge and a relative mass of 1. The relative charge of a proton is +1., neutral neutronUncharged subatomic particle, with a relative mass of 1. The relative charge of a neutron is 0 (neutral). and negative electronA subatomic particle with relative mass of ¹⁄₁₈₄₀. The relative charge of an electron is -1..
Protons and neutrons are located in the nucleusThe central part of an atom. It contains protons and neutrons, and has most of the mass of the atom. The plural of nucleus is nuclei., electrons orbit in shellAn energy level around the nucleus where electrons can be found orbiting.
Most of the mass and all of the positive charge of an atom is in the nucleus.
Most of an atom is empty space, (a vacuum).
What are atoms?
All matterSub-atomic particles and anything made from them, such as atoms and molecules, are matter. Energy and forces are not matter. is made up of atomAll elements are made of atoms. An atom consists of a nucleus containing protons and neutrons, surrounded by electrons., this includes all solids, liquids and gases.
Atoms are tiny particles that are too small to see, even with a microscope.
Experiments carried out by J.J. Thomson and Ernest Rutherford led physicists to believe that atoms themselves were made up of even smaller particles.
The plum pudding model
In 1897, the English born physicist, J J Thomson, was able to “rip” small negative pieces from neutral atoms using electricity.
He called this newly discovered particle the electronSubatomic particle, with a negative charge and a very small mass relative to protons and neutrons..
To explain how atoms were overall neutral, he proposed the “plum pudding model” in 1904.
He suggested that the atom consisted of negatively charged electrons randomly distributed in a positive doughIn the plum pudding model – dough means the sphere of positive material that it was thought the electrons were embedded within..
How did Rutherford discover the nucleus? (Higher tier only)
In 1906, a New Zealand-born British physicist, Ernest Rutherford, did an experiment to test the plum pudding model.
Around the same time, radioactivityThe process where certain material decay and emit one of 3 different types of radiation (alpha, beta or gamma). had been discovered.
Certain radioactive materials were observed to give off one of three type of radiation:
- alpha particles (positive)
- beta particle (negative)
- gamma rays
Rutherford’s two students, Hans Geiger and Ernest Marsden, directed a beam of positively charged alpha particles A particle comprising two protons and two neutrons (the same as a helium nucleus). at a very thin sheet of gold suspended in a vacuum.
As the alpha particles were known to have a lot of energy and the gold was very thin, it was predicted that the alpha particles should travel through the atoms without changing direction.
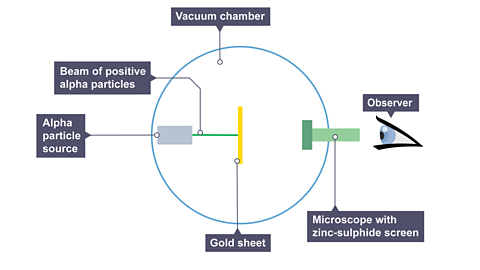
Image caption, A beam of positive alpha particles was directed towards a thin sheet of gold in a vacuum chamber. The vacuum was to avoid the alpha particles colliding with atoms in the air. When an alpha particle hits the zinc sulphide screen on the detector, a flash of light is observed. Geiger and Marsden counted these flashes of light at different angles around the vacuum chamber.
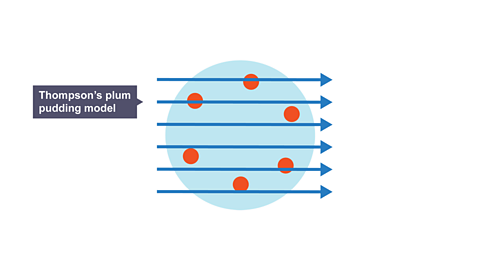
Image caption, Prediction: It was thought that as the alpha particles passed through plum pudding model, they would be pushed and pulled equally in all direction. Therefore, they all should pass straight through and hit the same point .
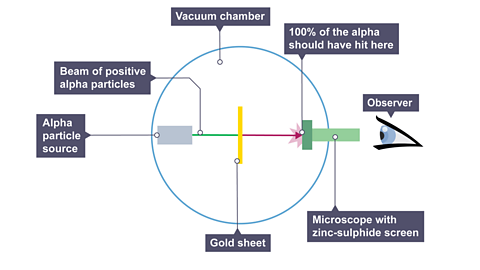
Image caption, Prediction: It was thought that as the alpha particles passed through plum pudding model, they would be pushed and pulled equally in all direction. Therefore, they all should pass straight through and hit the same point.
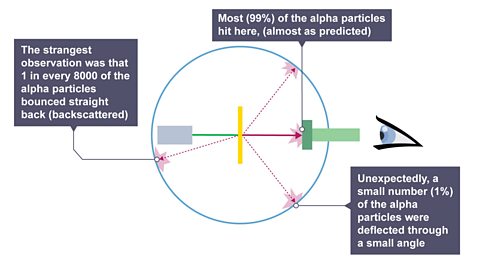
Image caption, 1. Most (99%) of the alpha particles hit the zinc-sulphide screen, (almost as predicted). 2. Unexpectedly, a small number (1 %) of the alpha particles were deflected through a small angle. 3. The strangest observation was that 1 in every 8000 of the alpha particles bounced straight back (“backscattered”).
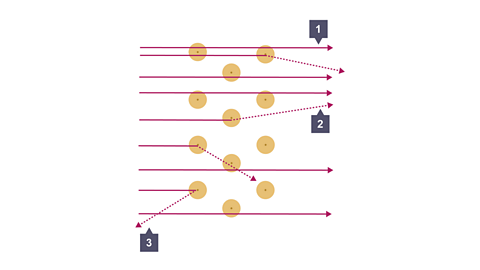
Image caption, At point 1, most of the alpha particles passed straight though. Conclusion: most of the atom is empty space.
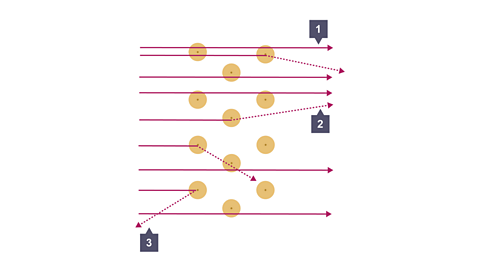
Image caption, At point 2, a small number, (1%), of the alpha particles were deflected if they got close to the centre of the atom. Conclusion: The centre of the atom is where all the positive charge is located. The nucleus had been discovered.
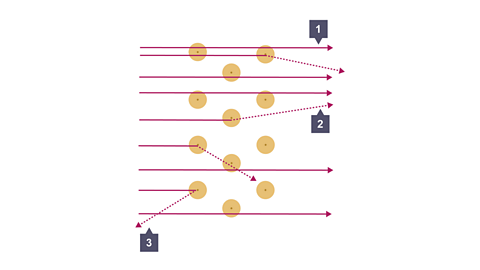
Image caption, At point 3, one in every 8000 of the alpha particles bounced straight back. (backscattered) Conclusion: The nucleus contains nearly all the mass of the atom in an incredibly small volume.
Image caption, Reaction: Rutherford was astonished by these results. He is reported to have said: “It was as if you fired a 15-inch shell at a sheet of tissue paper and it came back to hit you!”
1 of 8
Summary of the results of Rutherford's experiment
| Label | Observation | Conclusion |
|---|---|---|
| 1 | Most of the alpha particles passed straight though. | Most of the atom is empty space. |
| 2 | A small number, (1%), of the alpha particles were deflected if they got close to the centre of the atom. | The centre of the atom is where all the positive charge is located. The nucleus had been discovered. |
| 3 | One in every 8000 of the alpha particles bounced straight back. (backscattered). | The nucleus contains nearly all the mass of the atom in an incredibly small volume. |
What was Rutherford's conclusion?
Rutherford had discovered that atoms contain a nucleus, a small, positively-charged region surrounded by mostly empty space (a vacuumA volume of space that contains no matter.).
The discovery of the make-up of the nucleus (protonSubatomic particle with a positive charge and a relative mass of 1. The relative charge of a proton is +1. and neutronUncharged subatomic particle, with a relative mass of 1. The relative charge of a neutron is 0 (neutral).) came much later, and was not made by Rutherford.
The nucleus was calculated to be about \(\frac{1}{10000}\) the size of the atom.
Interesting fact: Virtually all the mass of atoms is in the nucleus and atoms contain so much empty space.
If you could remove all the empty space in atoms, the entire human population of the Earth would occupy a volume of 1cm3 (about the size of a sugar cube).
Further developments to the atomic model
Location of electrons – Bohr-Rutherford model.
Even though Rutherford had proven the existence of the nucleus, some issues still remained unanswered, for example how electrons fitted into this new model.
In 1913, Niels Bohr revised Rutherford's model by suggesting that the electrons orbited the nucleus in different energy levels, (called shellAn energy level around the nucleus where electrons can be found orbiting.).
This 'solar system' model of the atom is the way that most people think about atoms today.
It is known as the Rutherford-Bohr model of atomic structure.
The neutron was difficult to discover because it has no electrical charge but was needed to explain the different masses of different atoms.
It wasn't until 1932 that the English physicist, James Chadwick, was able to prove the existence of this neutral particle.
Summary of particles that make up the atom
| Particle | Relative mass | Relative charge | Location |
|---|---|---|---|
| Proton | 1 | +1 | In the nucleus |
| Neutron | 1 | 0 (neutral) | In the nucleus |
| Electron | \(\frac{1}{1840}\) | -1 | Orbiting the nucleus in shells. |
Question
The Rutherford-Bohr model of the atom is based on evidence obtained from alpha particleSubatomic particle comprising two protons and two neutrons (the same as a helium nucleus). scattering experiments using the apparatus shown below.
- Name the parts labelled A, B, C, D and E in the diagram above
- Describe how the experiment was carried out
- Briefly explain the finding from this experiment that 99% of particles were undeflected
- Briefly explain the finding from this experiment that a very small number of particles, (1 in 8000) are deflected more than 90°. (BackscatteredIn the Rutherford experiment, alpha particles that were deflected by an angle greater than 90°.)
Answer
- A - Alpha particle source; B – Thin sheet of gold foil; C – Moveable microscope with zinc sulphide screen; D –Vacuum ; E – Beam of alpha particles.
- The microscope is moved to different positions – rotating it 360° about the alpha particle source. At each angle the number of alpha particles detected (small flash of light observed) is counted and recorded.
- The atom is mostly empty space.
- This implies that the nucleus of the atom is positive and that most of the mass of the atom is concentrated in a small nucleus.
Test your knowledge
More on Unit 1: Atomic and nuclear physics
Find out more by working through a topic
- count2 of 6
- count3 of 6

- count4 of 6
- count5 of 6