What are the key learning points about bonding?
- There are three types of chemical bonding: ionic bondAn ionic bond is the attraction between oppositely charged ions., covalent bondA covalent bond is formed by a shared pair of electrons. and metallic bondThe attraction between delocalised electrons and the positive ions in a regular lattice..
- In ionic bonding, electronSubatomic particle, with a negative charge and a very small mass relative to protons and neutrons. are transferred from a metal atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons. to a non-metal atom to form oppositely charged ionElectrically charged particle, formed when an atom gains or loses electrons..
- In covalent bonding, non-metal atoms share electrons in order to achieve a full outer shellAn energy level around the nucleus where electrons can be found orbiting..
- In metallic bonding, metal atoms delocalise their outer electrons to form positive ions which are attracted to the delocalised electronsElectrons that are free to move throughout a whole structure..
What is bonding?
Bonding takes place when atoms join together.
There are three types of bonding studied at GCSE.
The type of bonding depends on the type(s) of elements that are being bonded together.
| Type of bonding | Elements that are being bonded |
|---|---|
| Ionic | Metal and non-metal |
| Covalent | Non-metal |
| Metallic | Metal |
What are ions?
Atoms tend to lose or gain electrons to form a full outer shell.
This is because having a full outer shell of electrons makes an atom stable.
For example, an oxygen atom has an electronic configuration of 2,6.
It can gain two electrons to form a stable full outer shell (the second shell holds a maximum of 8 electrons).
Atoms can also lose electrons to form a full outer shell.
For example, a sodium atom has a electronic configuration of 2,8,1.
It can lose one electron to be left with a full outer shell.
Key fact
Atoms will always choose the ‘easiest’ way to get a full outer shell.
For example, it is easier for a sodium atom to lose one electron to get a full outer shell rather than trying to gain an extra 7 electrons.
Anions and cations
As both the sodium atom and the oxygen atom no longer have the same number of protons and electrons they are no longer neutral and they have an overall charge.
They are now called ‘ions’ rather than ‘atoms.’
An ion is a charged particle.
They are formed when atoms gain or lose electrons to form a full outer shell.
- A negative ion is called an anion. An anion is formed when an atom gains electrons.
- A positive ion is called a cation. A cation is formed when an atom loses electrons.
A molecular ion is a particle made of more than one atom that has an overall positive or negative charge.
For example, the hydroxide ion, OH-, is a molecular ion.
Question
A magnesium atom and a fluorine atom are shown below.
Show how both atoms can form a full outer shell by gaining or losing electrons.
Work out the charge on the ions formed.
Answer
| The magnesium atom loses two electrons to get a full outer shell. | The fluorine atom gains one electron to get a full outer shell. |
| The magnesium ion has lost two negative electrons, so it now has a 2+ charge. It is a cation. | The fluoride ion has gained one negative electron, so it now has a - charge. It is an anion. |
Key facts
- When a non-metal atom turns into an ion, the element’s name changes to end in ‘-ide.’
For example, a fluorine atom turns into a fluoride ion.
- When writing the charge on the ion, remember to put the number before the positive or negative symbol (2+).
Just write + or – if the charge is 1+ or 1-.
What is ionic bonding?
Ionic bonding takes place between a metal and a non-metal.
For example, sodium (metal) and chlorine (non-metal) can form an ionic bond.
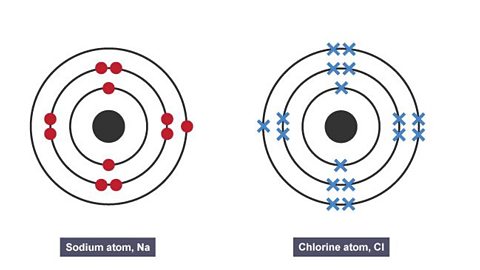
Image caption, 1. Sodium atom and a chlorine atom
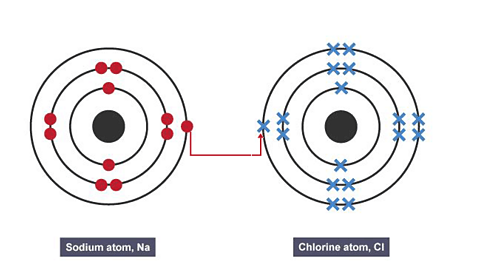
Image caption, 2. An electron is transferred from the sodium atom to the chlorine atom
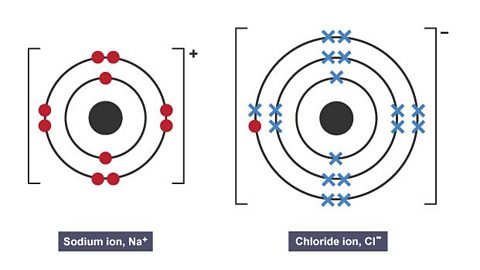
Image caption, 3. A sodium ion and a chloride ion are formed. They both have a stable full outer shell. The two ions have opposite charges and are held together by an electrostatic attraction.
1 of 3
To form a stable full outer shell, the sodium atom needs to lose one electron and the chlorine atom needs to gain one electron.
To achieve this, the sodium atom can transfer its outer electron to the chlorine atom.
This forms a sodium ion and a chloride ion that have opposite charges.
The two ions are held together by a strong electrostatic attraction. This is an ionic bond.
Summary of ionic bonding
When forming an ionic compoundA substance formed when two or more elements are chemically combined.:
- electronSubatomic particle, with a negative charge and a very small mass relative to protons and neutrons. are transferred from the metal atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons. to the non-metal atom.
- this forms oppositely-charged ionElectrically charged particle, formed when an atom gains or loses electrons..
- the metal ion has a positive charge.
- the non-metal ion has a negative charge.
- an ionic bond is the electrostaticA force that is caused by charged particles. attraction between these oppositely-charged ions.
- ionic bondAn ionic bond is the attraction between oppositely charged ions. is strong and requires substantial amounts of energy to break.
Ionic bonding
NARRATOR:
Ionic bonding — it's all about opposites attracting!
Not that kind of "opposites attracting"!
We're talking about charged particles, known as ions… Ions are formed when atoms lose or gain electrons.
And that makes them either positively or negatively charged.
Oppositely charged ions are attracted to each other by really strong electrostatic forces… forming what are known as ionic bonds.A good example of a substance containing ionic bonds is sodium chloride, or as we call it, table salt.
It's a compound of a metal and a non-metal, and it's made from positive sodium ions and negative chloride ions.
Ions form when electrons transfer from metal atoms to non-metal atoms.
The atoms of sodium, and other metals, have incomplete outer shells.
When they react with a non-metal, they lose all their outer electrons, so the shell below becomes the new outer shell — and it's complete.
It has no space for any other electrons.
An atom that loses an electron becomes a positively charged ion.
So, sodium atoms are written as Na, while sodium ions are written as Na⁺.
But the electrons must go somewhere when they leave metal atoms…
And they do — they're gained by non-metal atoms that have incomplete outer shells, making those shells complete.
The extra electrons turn the atoms into negatively charged ions. So, chlorine atoms are simply written as Cl, while chloride ions are Cl⁻.
And look! Now the ions have opposite charges — opposites attract and electrostatic forces pull them together.
That's your ionic bond right there!
Hello, table salt!
So, ions can form amazing structures — not just table salt.
In ionic compounds, the oppositely charged ions attract each other from all directions, forming solid structures known as giant ionic lattices.
Ionic bonds are really strong — it takes a lot of energy to break them, meaning ionic compounds have high melting and boiling points and are solid at room temperature.
Another property of these structures is that they don’t conduct electricity when they’re solid.
Why’s that? Well, an electric current is a flow of charged particles.
Solid ionic compounds don’t conduct because their charged particles — their ions — can’t move about.
They’re locked in place in the lattice structure.
If we want them to conduct electricity, we have to break the lattice up by melting or dissolving the ionic compound.
So there you go — the strength of opposites attracting…
Really!?
How to draw dot and cross diagrams to show ionic bonding
Dot and cross diagrams show how two elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. An element cannot be broken down into anything simpler by chemical means.ionic bondAn ionic bond is the attraction between oppositely charged ions..
There are 4 key steps involved in drawing dot and cross diagrams:
- Draw the electronic configurationThe order electrons are arranged in different shells. of each atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons. – one element with dots and the other with crosses.
- Work out how many electronSubatomic particle, with a negative charge and a very small mass relative to protons and neutrons. need to be transferred.
- Draw the electronic configuration of the ionElectrically charged particle, formed when an atom gains or loses electrons. that are formed.
- Write the charge of each ion.
Worked example
Use a dot and cross diagram to show the bonding between magnesium and oxygen.
Question
Draw a dot and cross diagram to show the bonding in calcium chloride.
Answer
In this case, a second chlorine atom is required, as the calcium atom needs to lose two electrons to form a full outer shell.
What is covalent bonding?
Covalent bonding occurs between non-metal atoms.
A covalent bond is formed when two atoms share electrons to form a full outer shell.
For example, two chlorine atoms can covalently bond to form a chlorine molecule.
Both atoms have 7 electrons in their outer shellAn energy level around the nucleus where electrons can be found orbiting..
Key fact
Only the outer shells of the atoms need to be shown in a dot and cross diagram for covalent bonding.
The outer shells of both chlorine atoms overlap and a pair of electrons is shared.
A covalent bond is a shared pair of electrons.
A covalent bond is strong and takes a large amount of energy to break.
Note the two types of electron pairs in the diagram:
- Bonding pair: a pair of electrons shared between two atoms.
- Lone pair: a pair of unshared electrons in the outer shell of an atom.
The Cl2 molecule is an example of a diatomic molecule – two atoms covalently bonded.
Here are the dot and cross diagrams for some common molecules.
Key fact
A covalent bond can be represented by a line (–) in a structural formula.
Covalent bonding
NARRATOR
Luckily for us, atoms don't always just sit around.
They can stick to other atoms and create amazing things — like the salt and vinegar on your chips. So how do they do it?
Well, one of the main ways is through covalent bonding.
It occurs in most non-metal elements, and it's how atoms bond to form molecules — and these can be elements, or compounds (made up of two or more different elements).
So, what brings these atoms together?
Electrons.
Most atoms have outer shells of electrons that aren't completely filled up — they've still got room for some more…
If they share electrons, they can fill up their outer shell — and form molecules.
And when two atoms get all cosy with each other, and share a pair of electrons between them, that's a covalent bond.To draw covalent bonds, we use dot and cross diagrams.
For instance — carbon dioxide consists of one carbon atom bonded to two oxygen atoms.
Now, carbon is in group 4 of the periodic table, so it has four electrons in its outer shell.
And for that outer shell to be completed, it needs four more.
Oxygen is in group 6, so — you've guessed it — it has six electrons in its outer shell.
So, when forming carbon dioxide, each oxygen atom shares two electrons, forming a double covalent bond with a carbon atom.
When that happens, the outer shell of the carbon atom is completed — four of its own electrons and two from each of the oxygen atoms.Do note that both oxygen atoms still have four non-bonding electrons that are just left over.
Now — there's a difference between simple covalent structures, and something called giant covalent structures.
Methane, the gas that's used in your Bunsen burners, exists as simple covalent molecules.
That means the atoms in each individual methane molecule are held together by covalent bonds —but there's not much holding one molecule to another — only weak intermolecular forces.
And it doesn't take much energy to overcome them.
On the other hand, giant covalent structures are made up of a huge number of atoms, all joined by covalent bonds in continuous 3D networks.
For example, let's have a look at diamond.
That’s made of carbon atoms, and each carbon atom is bonded to four other carbon atoms to form a continuous regular lattice.
Covalent bonds are strong — and there are loads of them in diamond, making it the hardest natural substance in the world!
And giving it a ridiculously high melting point.
Graphite is another giant covalent substance.
And though it's made from the same stuff as diamond — it is nothing but carbon atoms — it's really quite different — it's all soft and slippery.
Why?
Well, in graphite, each atom is bonded to three, not four, other carbon atoms.
They end up forming layers with only weak intermolecular forces between them — making it all slippery and crumbly.
The carbon atoms in graphite don’t share all their outer electrons, so they have non-bonding outer electrons.
These electrons are free to move around the lattice, which means graphite can do something rather useful that diamond can't —it can conduct electricity.
So there can be a fair bit of difference in the way covalent structures are formed.
But they all contain those strong covalent bonds, which of course are just atoms, sharing electrons…
Well, sharing is caring.
What are multiple covalent bonds?
In some cases, more than one pair of electronSubatomic particle, with a negative charge and a very small mass relative to protons and neutrons. can be shared between two atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons..
In an oxygen molecule (O2), two pairs of electrons are shared between the oxygen atoms.
This is a double covalent bond.
There are three different kinds of covalent bonds:
- a single covalent bond is when two atoms share a single pair of electrons. Represented by a single line (–).
- a double covalent bond is when two atoms share two pairs of electrons. Represented by a double line (=).
- a triple covalent bond is when two atoms share three pairs of electrons. Represented by a triple line (≡).
The table below shows several molecules with double and triple covalent bonds.
What is metallic bonding?
In a metal, atoms are able to form a full outer shell by delocalising their outer electrons.
This means the outer electrons are released from the atom and can move freely.
When lots of metal atoms delocalise their outer electrons, a regular latticeA regular grid-like arrangement of atoms in a material. of positive ionElectrically charged particle, formed when an atom gains or loses electrons. is formed, surrounded by delocalised electrons.
Metallic bonding is the attraction between the positive ions in a regular lattice and the delocalised electrons.
Delocalised electrons are free to move throughout the whole structure, they are often described as a delocalised sea of electrons.
When drawing a diagram of a metal’s structure, be sure to draw the ions in regular rows.
How much do you know about bonding?
More on Unit 1: Structures, trends, chemical reactions, quantitative chemistry and analysis
Find out more by working through a topic
- count3 of 10

- count4 of 10
- count5 of 10
- count6 of 10
