What are the key learning points about the periodic table?
- Dmitri Mendeleev developed an early version of the periodic table that places elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. An element cannot be broken down into anything simpler by chemical means. with similar properties in the same vertical group.
- The alkali metals (Group 1) are a group of highly reactive metals that have distinctive observations when they react with water.
- The halogens (Group 7) are a group of highly reactive non-metals that can react with each other in displacement reactionA reaction where a more reactive element displaces a less reactive element from its compound..
- The noble gases (Group 0) are a group of non-reactive gases, and the transition metals are a block of metals that react to produce colourful compoundA substance formed when two or more elements are chemically combined..
What is the history of the periodic table?
An element is a substance that is made up of only one type of atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons., that cannot be broken down into simpler substances by chemical means.
In the 1800s, many chemists attempted to organise the elements into a logical order.
However, at this time most of the elements had not yet been discovered, so these chemists’ tables were incomplete, and often had mistakes in them.
What was Mendeleev’s periodic table?
In 1869, the Russian chemist, Dmitri Mendeleev published a periodic table that was the starting point for the table we use today.
Mendeleev’s periodic table had three important features:
- He arranged the elements in order of increasing atomic mass.
- He put elements with similar properties in the same group.
- He left gaps for undiscovered elements.
What does the modern periodic table look like?
Scientists have made slight changes to Mendeleev’s periodic table over time:
- The modern periodic table is arranged in order of increasing atomic number, rather than atomic mass.
- There are no gaps in the modern periodic table as more elements have been discovered.
- The noble gasesThe elements in Group 0 of the Periodic Table, named for their lack of chemical reactivity. were discovered and added as a group to the modern periodic table.
- The modern periodic table includes actinides and lanthanides.
- The transition metalsA metal that is located in between Group 2 and Group 3 of the periodic table and has brightly coloured compounds. are arranged in a separate block in the modern periodic table.
Structure of the periodic table
What are groups and periods?
The modern table is called a “periodic” table because the elements with similar properties appear at regular intervals.
- The vertical columns are called groups.
- The horizontal rows are called periods.
What are metals and non-metals in the periodic table?
There are many divisions in the periodic table, but one of the most important is between the metals and the non-metals.
The metal elements are on the left of a ‘stepped line’ that runs below boron (B), silicon (Si), arsenic (As), tellurium (Te), and astatine (At).
You can tell the difference between the metals and non-metals by looking at their properties.
| Properties of a typical solid metal | Properties of a typical solid non-metal |
|---|---|
| Good conductor of electricity. | Poor conductor of electricity. |
| Good conductor of heat. | Poor conductor of heat. |
| Generally high melting points. | Generally low melting points. |
| Malleable (can be hammered into shape). | Brittle (breaks when hammered). |
| Ductile (can be drawn out into wires). | Brittle (snaps when stretched). |
| Sonorous (makes a ringing sound when struck). | Not sonorous. |
What is the state of the different elements at room temperature?
The non-metal elements hydrogen nitrogen, oxygen, fluorine, chlorine and all the noble gases (Group 0) are gases.
Bromine and mercury are the only liquid elements.
Every other element is a solid.
What does a group number mean?
Elements in the same group have the same number of electronSubatomic particle, with a negative charge and a very small mass relative to protons and neutrons. in their outer shell.
For example, every element in Group 1 has 1 electron in the outer shellAn energy level around the nucleus where electrons can be found orbiting..
You need to know the names of the following groups:
| Group | Name |
|---|---|
| 1 | Alkali metals |
| 2 | Alkaline earth metals |
| 7 | Halogens |
| 0 | Noble gases |
What are the key properties of Group 1 – the alkali metals?
The Group 1 elements all have similar physical properties:
- Low density - the first three (lithium, sodium and potassium) can all float on water.
- Very soft - they are easily cut with a knife.
- Shiny when first cut, but tarnish rapidly in air.
- Low melting points.
How do Group 1 metals react with water?
All alkali metals react with water to produce a metal hydroxide and hydrogen.
metal + water→ metal hydroxide + hydrogen
For example, here is the reaction of sodium with water:
sodium + water → sodium hydroxide + hydrogen
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
This reaction is quite dangerous as the Group 1 metals are all highly reactive.
They are stored under oil to prevent any reaction with air or water vapour when in storage.
The following observations are made for the reaction of lithium, sodium and potassium with water:
| Metal | Observations |
|---|---|
| Lithium | • Metal floats on surface of water. • Metal moves about on the surface. • Fizzing. • Heat is released. • Metal disappears. • Colourless solution is formed. |
| Sodium | Same observations as lithium, but also: • Metal melts into a ball. • Sometimes an orange flame is observed. |
| Potassium | Same observations as lithium, but also: • Metal melts into a ball. • Lilac flame produced. • Crackling noise heard. |
The following safety precautions must be followed when carrying out the reaction of Group 1 metals with water:
- Use tweezers when lifting alkali metals.
- Use a safety screen and wear safety glasses.
- Use a small piece of metal.
- Use a large volume of water (eg in a trough)
Group 1 metals all react in the same way, because they all have one electron in their outer shell.
This means each of them loses an electron to form a positive ionElectrically charged particle, formed when an atom gains or loses electrons. with a stable full outer shell.
For example, sodium loses one electron to form a sodium ion, Na+, with a full outer shell.
This can be represented by a half equationAn equation, involving ions and electrons, that describes the process happening at an electrode. (higher tier only):
Na → Na+ + e-
Group 1 metals become more reactive as you move down the group.
Elements further down a group have larger atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons., and the larger the atom, the further the outer electronSubatomic particle, with a negative charge and a very small mass relative to protons and neutrons. is from the nucleusThe central part of an atom. It contains protons and neutrons, and has most of the mass of the atom. The plural of nucleus is nuclei..
The force of attraction between the nucleus and the electron is less than for smaller atoms, and the outer electron is lost more easily.
What are the properties of Group 7 (VII) – the halogens?
Group 7 elements exist as simple, diatomicTwo atoms covalently bonded. molecules.
Each molecule is made up of a pair of halogensThe non-metal elements which are in group 7 of the periodic table (second group from the right). atoms, linked by a single covalent bondA covalent bond is formed by a shared pair of electrons..
Halogens are toxicPoisonous, harmful..
You should use a fume cupboard if you are using them in experiments.
| Halogen | Formula | Colour | State at room temperature and pressure |
|---|---|---|---|
| Fluorine | F2 | Yellow | Gas |
| Chlorine | Cl2 | Yellow-green | Gas |
| Bromine | Br2 | Red-brown | Liquid |
| Iodine | I2 | Grey-black | Solid |
Key fact
You will notice that, moving down Group 7, the elements become darker in colour and change from gas to liquid to solid.
This is because elements lower in the group have higher melting points.
What is iodine sublimation?
Iodine is a grey-black solid at room temperature and pressure.
If heated, it sublimes to form a purple gas.
Sublimation is the change of state from solid directly to gas on heating, without passing through the liquid phase.
What is the test for chlorine gas?
Damp universal indicator paperPaper stained with universal indicator, a chemical solution that produces many different colour changes corresponding to different pH levels. changes to red and then bleaches white in the presence of chlorine gas.
What are Group 7 (VII) displacement reactions?
Halogen elements can react with each other in a reaction called a displacement reaction.
This involves a reaction where a more reactive halogen displaces (pushes out) a less reactive halogen from a compound.
For example, chlorine can react with potassium bromide.
In this reaction, just the bromine and the chlorine react (the potassium is just a spectator ionAn ion that is present in a reaction but does not take part. Its formula will be the same on both sides of the equation.).
Chlorine is a more reactive halogen than bromine, so it is able to displace bromine from its compound and take its place.
Click through the slides below to see the equations and observations for this reaction.
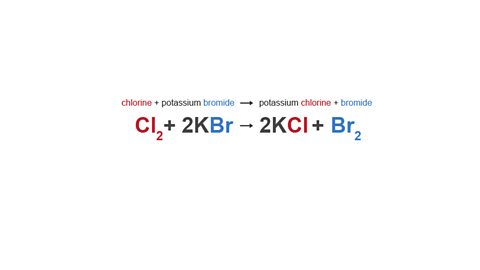
Image caption, Equation
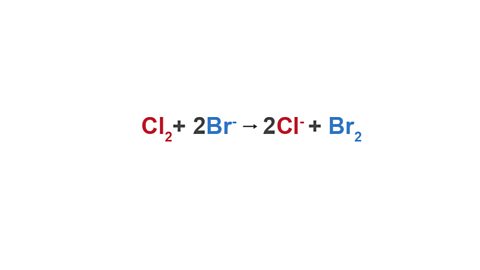
Image caption, Ionic equation (higher tier only)
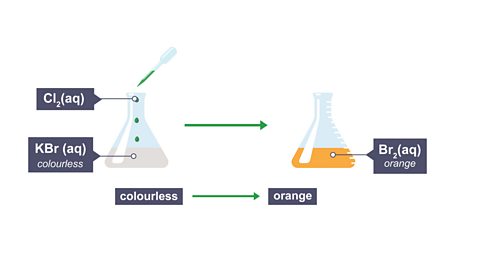
Image caption, Observations
1 of 3
Chlorine can also displace iodine from a compound.
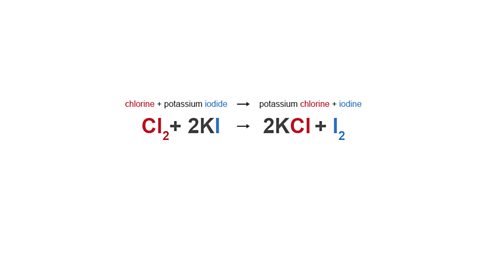
Image caption, Equation
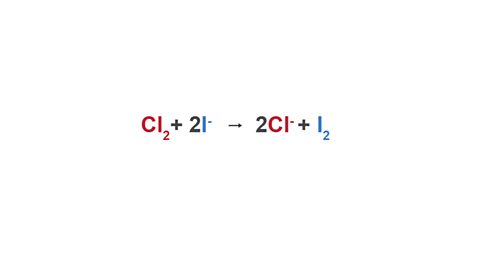
Image caption, Ionic equation (higher tier only)
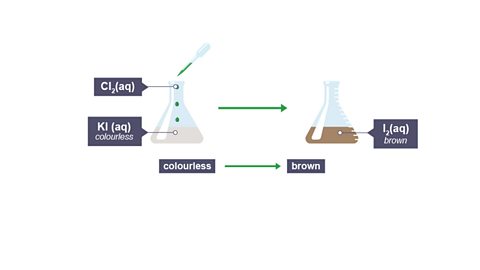
Image caption, Observations
1 of 3
Question
Bromine (Br2) can react with a solution of potassium iodide (KI) in a displacement reaction.
What is the balanced symbol equation for this reaction, and what are the observations?
Answer
Equation: Br2 + 2KI → 2KBr + I2
Observations: colourless solution (KI) turns brown (I2 in solution).
What is the reactivity of the halogens?
When a Group 7 element reacts, it gains one electronSubatomic particle, with a negative charge and a very small mass relative to protons and neutrons. to form a negative ionElectrically charged particle, formed when an atom gains or loses electrons. with a stable full shellAn energy level around the nucleus where electrons can be found orbiting..
As we move down the column of Group 7 elements, their reactivity decreases.
In each, the outer shell is further from the nucleusThe central part of an atom. It contains protons and neutrons, and has most of the mass of the atom. The plural of nucleus is nuclei. and the incoming electron is not as easily attracted to it.
half equationAn equation, involving ions and electrons, that describes the process happening at an electrode. can be used to represent these reactions (higher tier only):
Chloride ions from a chlorine molecule:
Cl2 + 2e- → 2Cl-
A chloride ion from a chlorine atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons.:
Cl + e- → Cl-
What are the properties of Group 0 – the noble gases?
Group 0 elements have stable electronic configurations – full outer shells of electrons – and are therefore unreactive.
The Group 0 elements are all colourless gases.
All noble gases have low boiling points.
Their boiling points increase as we move down the group.
What are the properties of the transition metals?
The transition metals are in the block in the middle of the periodic table, between Groups 2 and 3.
They are all metals and include many common metals such as chromium (Cr), iron (Fe), nickel (Ni) and copper (Cu).
The properties of the transition metals are often quite different to those of the Group 1 metals.
This is shown in the table below.
| Physical properties | Group 1 – Alkali metals | Transition metals |
|---|---|---|
| Melting point | Low melting point | High melting point (except mercury) |
| Density | Low density – Li, Na and K are less dense than water | High density |
| Reactivity with water | Very reactive with cold water | Low reactivity with water |
| Formation of ionElectrically charged particle, formed when an atom gains or loses electrons. | React to form 1+ ions (e.g. K+) | React to form ions with different charges e.g. iron can form Fe2+ and Fe3+ ions |
| Colour of compoundA substance formed when two or more elements are chemically combined. | Form white compounds, e.g. sodium chloride is white | Form coloured compounds (e.g. copper(II) carbonate is green) |
You will notice under ‘formation of ions’ that the transition metals react to form ions with different charges.
An iron(II) ion has a 2+ charge, and an iron(III) ion has a 3+ charge.
Transition metals tend to form coloured compounds:
| Transition metal compound | copper(II) oxide | copper(II) carbonate | hydrated copper(II) sulfate | any copper(II) salt in solution |
|---|---|---|---|---|
| Colour | Black solid | Green solid | Blue crystals | Blue solution |
How much do you know about the periodic table?
More on Unit 1: Structures, trends, chemical reactions, quantitative chemistry and analysis
Find out more by working through a topic
- count7 of 10

- count8 of 10

- count9 of 10

- count10 of 10
