Dissolving

When a substance dissolves, it might look like it has disappeared, but in fact it has just mixed with the water to make a transparent (see-through) liquid called a solution.
Substances that dissolve in water are called soluble substances.
When you mix sugar with water, the sugar dissolves to make a transparent solution.
Salt is soluble in water too.
Substances that do not dissolve in water are called insoluble substances. For example, when you mix sand or flour with water, they do not dissolve.

Watch: What is dissolving?
Learn about dissolving and mixing.
Time for a drink?
Great, I'm a bit parched.
Some substances dissolve when you mix them with water like sugar or jelly or salt…
Well that's gross!
It looks like it's disappeared but it's still there.
It's just mixed with the water to make a transparent liquid called a solution.
A substance that is able to dissolve in water is known as a soluble.
Eurgh, that can't have tasted good.
However, when you mix other substances with water they don't dissolve.
These are called insoluble substances.
They just stay the same in water and sink to the bottom. Like sand.
You can see the sand in the water so nobody would drink that.
Oh…that is…that is just disgusting.
Fascinating facts

Fascinating facts about dissolving!
Dissolving is a reversible change.
Temperature affects the rate at which a soluble substance dissolves.
Stirring faster can also increase the rate at which a soluble substance dissolves through the addition of kinetic (motion) energy.
We call the degree to which something dissolves it's solubility.
Soluble substances include salt, soap and sugar.
Insoluble substances include plastics, wood and metal.

When you add some sugar to water and stir, what does the water taste like? Can you explain why?
The water tastes sweet because the sugar is still there, even if you can’t see it because it has dissolved.
Dissolving

When a substance dissolves, it might look like it has disappeared, but in fact it has just mixed with the water to make a transparent (see-through) liquid called a solution.
Substances that dissolve in water are called soluble substances. When you mix sugar with water, the sugar dissolves to make a transparent solution.
Salt is soluble in water too.
Substances that do not dissolve in water are called insoluble substances. For example, when you mix sand or flour with water, they do not dissolve.

Reversing dissolving
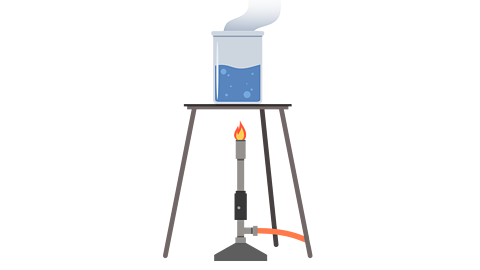
Dissolving is a reversible change, which means that you can reverse the dissolving process to get the original solute back.
This is done by heating up the solvent until it reaches its boiling point and turns into a gas, this is called evaporation.
Once all the liquid has evaporated you are left with the solute that you started with.

Watch: Solvents and solutions
Learn all about solvents and solutions.
Narrator: A trip to the seaside is always fun. Even in winter.
But it does get a bit nippy. And we need to warm up! A mug of hot chocolate that’ll do the trick. And it’ll help us understand solutions.
Solutions are simply one thing dissolved in another. You need a solute, that’s the substance being dissolved. And a solvent, that is the substance that does the dissolving.
Salt and sugar are great examples of solutes, and water is a great example of a solvent. Salt and sugar both dissolve in water to make a solution.
Solvents can only dissolve a certain amount. When it can’t dissolve any more, the leftover solute will stay in the liquid as a solid.
It’s also possible to get back a solute that has been dissolved. Just boil the water. As the boiling water turns into steam and evaporates, the solid is left behind.
And then there are things that’s don’t dissolve at all. Sand is one of them. Pepper is another… it doesn’t dissolve… oh wait a minute… aaahhh ATCHOOOOOO!
Oh! Thanks Cara!

Did you know?
The Dead Sea which is bordered by both Jordan and Israel, is nine times saltier than the ocean.
Like all seawater, the salt is soluble and has dissolved in the water.
The water in the Dead Sea is so salty there is very little animal or plant life that can survive in it. It is also naturally very buoyant, meaning that you can easily float on the surface.

Slideshow: Soluble and insoluble

Image caption, Soluable
Salt is a soluble substance which means it dissolves in liquids. Salt can be retrieved after it's dissolved by using evaporation. This is called a reversible change.

Image caption, Insoluble
Plastic is a man-made material, used to make many things like fishing nets which do not dissolve in liquid. This is called an insoluble substance.
1 of 2

Did you know?
You don't just have to wait for a substance (the solute) to dissolve, you can help it to dissolve faster by making the liquid (the solvent) hotter.
Stirring the solvent can also help to dissolve the solute faster because it helps to break up the solute into smaller pieces more quickly.

Dissolving or melting?
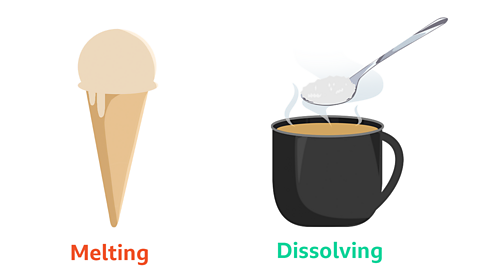
If you are using a hot solvent, such as hot water for coffee or hot chocolate, you might think that the solute that you are adding is melting.
However melting and dissolving are two different processes.
While you need heat for something to melt, dissolving can happen with or without heat.
Melting is when something changes state. This means it changes from a solid to a liquid, or a liquid to a gas when it is heated.
Dissolving is when something has been broken down into smaller particles and mixed with another substance to create a solution.
When you add sugar into a hot cup of tea the sugar doesn't melt because it doesn't change state from a solid into a liquid. It dissolves because the sugar is still in the solution. The sugar has just been broken down into tiny particles that have mixed in with the hot solvent, the tea.
If you added sugar to cold tea it would still dissolve but it would take much longer.

Watch: Dissolving and evaporation
Here's what you needed to know about dissolving and evaporation.
Narrator: Some substances dissolve when you mix them with water. This is called a solution.
If you take salt and mix it with water, it might look like the salt has disappeared. But it’s actually dissolved in the water.
Dissolving is a physical change, because salt combines with the water to form a solution.
We can also reverse it. If you leave the salty solution in a sunny spot, the water evaporates, it has changed into water vapour, a gas, leaving behind solid salt.

Did you know?
You cannot add an unlimited amount of a solute to a solvent.
If you add sugar to a glass of water the sugar will keep dissolving until the water reaches its saturation point (the maximum amount of the solute that the solution can dissolve).
After this, if you keep adding more sugar you will see it begin to settle in the bottom of the glass.

Important words
Dissolve – When a solid substance has mixed with a liquid to make a transparent (see-through) liquid.
Evaporation – When a liquid turns into a gas slowly, at temperatures below its usual boiling point.
Insoluble – Any substance that does not dissolve in water.
Melting – When something changes state from a solid to a liquid by using heat.
Mixture – Something that is created when you combine two or more things together.
Reversible change – A change that can be undone or reversed.
Separate – To split two substances up from one original substance.
Soluble – Substances that dissolve in water.
Solution – A type of mixture which is created when you dissolve a solid (or a liquid) into a liquid.
Substances – Any solid, liquid or gas with its own properties.
Solvent – A substance that dissolves other substances.

Activities
Activity 1 – Explore the hotspots
Tap on the different substances to find out what happens when you add them to water.
Activity 2 – Fill in the gaps
Activity 3 – Sort the pictures
New game! Horrible Science: Stinky Space. gameNew game! Horrible Science: Stinky Space
Join Pipette on her epic mission and learn some revolting facts about space along the way.

More on Properties and change of materials
Find out more by working through a topic
- count4 of 5
- count5 of 5

- count1 of 5

- count2 of 5

