Key points
- Solids, liquids and gases change state when they are heated or cooled.
- Processes such as evaporation and boiling change the state of substances.
- A particle model can be used to show how solids, liquids and gases change state.
Video
Watch this video to see how a chef uses their understanding of changes of state while they are cooking.
I have some frozen coconut. And this forms the base of our favourite sauces.
What we need to do now is just pop this into the pan and you can see it starts to melt straight away.
Now you can see that the block of coconut is melting really quickly and as the heat gets to it, it will thicken and bubble and it will get really creamy.
As the chef heats the liquid coconut milk, bubbles can be seen in the pan. What is happening to the liquid?
The liquid is boiling.
Changes of state
When an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil.meltingWhen a solid turns into a liquid as it absorbs energy from the surroundings. and boilingWhen a liquid turns into a gas as it absorbs energy from the surroundings. are changes of state, and they can occur in reverse too if a substance is cooled down.
Video
Watch this video to see how energy is involved in a substance's changing state.
Energy in changes of state.
Substances can change states. From solid to liquid to gas and back again. And this usually happens when they are heated or cooled.
It's Keisha’s birthday and her dad has baked a cake and wants to cover it with chocolate. He puts a solid chocolate bar in a bowl and warms it over hot water. As the chocolate heats up it begins to melt. The chocolate is absorbing energy from its surroundings. The heat from the water below. This is known as an endothermic process, where heat is absorbed during the change of state.
The chocolate is now a liquid and Keisha’s dad is able to pour it over the cake. After a few hours, Keisha notices that the chocolate is now solid, its state has changed back again. This is an exothermic process. The heat energy that the chocolate absorbed is released, causing it to cool down and become solid.
Keisha’s dad lights her birthday candles. Better blow them out before the candles melt.
What is the process used to melt the chocolate?
Heating is used to melt the chocolate. When a solid is heated, it absorbs energy and it melts, turning into a liquid.
Heating up and cooling down
Substances can change state when they are heated or cooled.
Heating up
- Melting - When a solid is heated, it absorbs energy and it melts, turning into a liquid.
- Boiling - If the liquid is heated, it absorbs more energy and it boils, turning into a gas.
- These changes absorb energy from the surroundings so they are endothermicA physical change or chemical reaction that absorbs energy from the surroundings..
- Evaporating is when a liquid turns into a gas slowly, at temperatures that are below its boiling pointThe temperature at which a pure substance boils from a liquid into a gas. For example, the boiling point of pure water is 100°C. The boiling point is also the temperature at which a gas will condense into a liquid.. Puddles dry up because they evaporatingWhen a liquid turns into a gas slowly, at temperatures below the boiling point. – they don’t boil.
Cooling down
- Condensing - If a gas is cooled, it transfers energy to the surroundings, and turns into a liquid.
- Freezing - If the liquid is cooled, it transfers energy to the surroundings, and turns into a solid.
- These changes transfer energy to the surroundings so they are exothermicA physical change or chemical reaction that transfers energy to the surroundings..

- Endo sounds like ‘into’ - the energy goes into the chemicals from the surroundings.
- Exo sounds like ‘exit’ - the energy is leaving the chemicals and is transferred to the surroundings.
What is the opposite change of state to condensing?
Boiling is the opposite of condensing. During condensing, a gas changes into a liquid. During boiling, it is the other way round.
How water changes state
This diagram shows the processes that take place when water changes state.
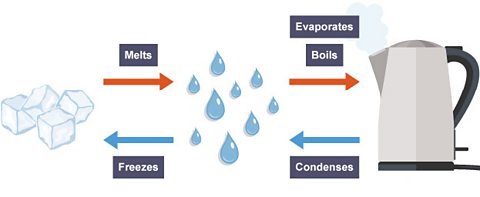
Which changes of state occur when a substance is cooled down?
Condensing and freezing.
Arrangement of particles
Video
Watch this animation to see how the arrangement and behaviour of particles in a solid change when heated.
Models for atomic structure for solid, liquid and gas
When a liquid is heated, it boils. Describe what happens to the movement and arrangement of the particles during boiling.
When heated, the particles gain energy and become widely spaced. When the particles have gained enough energy, they move much faster in straight lines breaking away from the other particles. When a liquid is boiling, some of its particles enter the gas state.
Ice
Some solids change directly into a gas without becoming a liquid first. This process is called sublimation.
Solid carbon dioxide is often called dry ice. It must be kept inside well insulated containers because when its temperature rises above -78 °C it sublimingWhen a solid turns into a gas without becoming a liquid first. Subliming is also called sublimating., or sublimates, into carbon dioxide gas. This process is endothermicA physical change or chemical reaction that absorbs energy from the surroundings., so it can be used to keep other substances cold.

Dry ice must be handled carefully because it can cause frostbite if touched, which means it will freeze skin and body tissues. Dry ice can also be used to create artificial fog in theatres.

Video
Watch this video about another useful change of state, making ice cream using a freezing mixture of salt and ice.
Teacher: I come armed with everything we need to have a tasty chemistry lesson.
Presenter: This is already the best chemistry class I've ever been in.
Teacher: Will it impress you even more if I said we will be making ice cream without a freezer?
Presenter: Whoa! Hang on, actual edible ice cream that is fully frozen without a freezer?
Teacher: Absolutely. To make your very own instant ice cream at home we will need a large and small zip lock bag, ice cubes, salt, your favourite flavoured milk, a bowl or cone, warm gloves and, if you want, some toppings.
Time for the recipe.
First, I poured half a cup of flavoured milk into the small zip lock bag and sealed it tight. I filled a large zip lock bag with ice and food colouring so you can see it better at home. Now I will add six tablespoons of salt.
Presenter: Why do you do that? Salt, ice cream… What?
Teacher: The salt lowers the freezing point of the ice and, if you look closely, you will see the ice begin to melt. You can see this science in action when salt is spread on roads and pavements in freezing weather. It melts the ice, making it safe to drive or walk on.
Carefully place the small zip lock bag inside the large bag with the ice and salt and seal it all up nice and tight.
Put on some gloves so your hands don't get too cold.
Presenter: Oh, important question! Very important. Would mittens do?
Teacher: Yes, I think they will.
Now, gently shake and squeeze the bag. Can you see what's happening to the ingredients?
Presenter: Yeah, it's getting a little thicker.
Teacher: The addition of the salt has made the ice a few degrees below freezing. The water in the milk changes state and turns from a liquid into a solid, turning it into delicious ice cream.
Presenter: Ah, very clever. So, how long do you need to keep doing that?
Teacher: You need to shake it for about five minutes and, once you're ready, remove the small bag and put the ice cream into a bowl or cone. Add your toppings and it should look something like this.
Presenter: Ah, that looks like proper ice cream!
Working scientifically
Working safely
When investigating changes of state, we often use heating apparatus like a Bunsen burner. It’s important to follow some sensible safety rules when heating chemicals.

- Wear eye protection just in case something hot splashes into your face.
- When using a flame, tie long hair back.
- Make sure to work standing up, so if you spill anything hot it will land on the table or bench, not on your legs.
- Use the appropriate apparatus to hold anything hot. A test tube holder or clamp stand can be used to hold a test tube when it is heated or hot.
Find out more about working safely in science in this working safely guide.

Test your knowledge
Teaching resources
Looking for resources for your chemistry lessons? In this series of short films rapper Jon Chase uses magic, music and more to bring science to life.
BBC Bitesize for Teachers has thousands of free, curriculum-linked resources to help deliver lessons - all arranged by subject and age group.
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on The particle model of matter
Find out more by working through a topic
- count3 of 4

- count4 of 4

- count1 of 4
