Solutions and separations
There are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. The method chosen depends upon the type of mixture.
Solubility with Jon Chase
I've got a question for you guys. Do you think I can fit this length of polystyrene into this jar? Yes. No. No? Yeah? I like that – you lot have got some faith in me! Excellent. Right, this is what we're going to do… We're going to take this length of polystyrene and stick it into this Pyrex jug. But we're going to use something to help us do it. It's this stuff. It's called propanone, or acetone. We're going to dissolve this polystyrene into this.
When we dissolve it in, this will be called the solute, and this stuff, that does the dissolving, will be called the solvent. And when they're mixed together, they will be called a solution. You add a bit of the solvent… Something's happening. Slowly but surely, it's going in. So, remember, acetone's the stuff that you've actually got in nail varnish remover. Aw, yeah! Swill that around. Hurray! One, two, three…! Magic! Oh, yes. So there we have it, a whole polystyrene rod fitted into a little Pyrex jar.
Hi. Can I have two cups of tea, please? Sure. Solubility is a measure of how much solute can dissolve in a solvent. The solubility of a solute in a solvent changes with temperature. And importantly, it depends on whether the solute is a gas or a solid. So, let's look at solids first. Here we have two identical hot cups of tea. And we want to see how much sugar can be held in solution in these hot cups of tea. When no more sugar can dissolve, the solution is said to be saturated. I think that's getting just about saturated now.
But the situation changes when the liquid is cooled down. Luckily, I've got a bit of dry ice here, and that should do the job perfectly. Dry ice is at a temperature of minus 78 degrees centigrade, so it's going to cool the water down. In this one a tiny bit of sugar has crystallised at the bottom because it's a bit cooler. But this one, look how much sugar is actually in the bottom. The sugar behaves like most solids – the solubility increases as the temperature of the solvent does. What's interesting about gases is that they behave in the opposite way to solids. The solubility of gases decreases as the temperature increases.
I'm going to show you a neat trick. Check out these ice cubes. One set's lovely and clear, but the other one's pretty cloudy. It's all down to the solubility of gases in a liquid. So to make cloudy ice cubes, all we need to do is take tap water and put it straight in. But if we want our ice cubes to be clear, you have to boil the water first. And here's why boiling the water makes a difference. At room temperature, the water contains a certain amount of dissolved gases from the air. The water straight from the tap creates cloudy ice cubes because these gases that were dissolved in the water form tiny bubbles in the ice.
By heating the water to boiling point, we have decreased the solubility of the dissolved gases. They come out of the solution as bubbles and the remaining water has less gases dissolved, so is less cloudy. For a solution, the solubility of gases decreases as we increase the temperature.
Game - separating salt and sand
Play an Atomic Labs experiment exploring how to separate salt and sand.
You can also play the full game
(Practical advice on how to work safely in the lab when conducting science experiments.)
Separation
Mixtures can be easily separated. Methods like sieving, filtering, chromatography and evaporating can be used.
Sieving
A mixture made of solid particles of different sizes, for example sand and gravel, can be separated by sieving.

Separating a mixture of iron filings and sand
A mixture of iron filings and sand can easily be separated using a magnet. The iron filings are attracted to the magnet, but the sand is not. A mixture of iron filings and sulfur powder could be separated in exactly the same way.

Filtration
Remember
| Term | Meaning | Example |
|---|---|---|
| Solute | The substance that dissolves | Sugar |
| Solvent | The liquid the substance dissolves in | Water |
| Solution | The liquid mixture of solute and solvent. Solutions are clear – you can see through them | Sugar water |
| Soluble | A substance that can be dissolved in a solvent | Salt is soluble in water |
| Insoluble | A substance that cannot be dissolved in a solvent | Sand is insoluble in water |
Filtration is a method for separating an insoluble solid from a liquid. When a mixture of sand and water is filtered:
- the sand stays behind in the filter paper (it becomes the residue)
- the water passes through the filter paper (it becomes the filtrate)
The slideshow shows how filtration works:
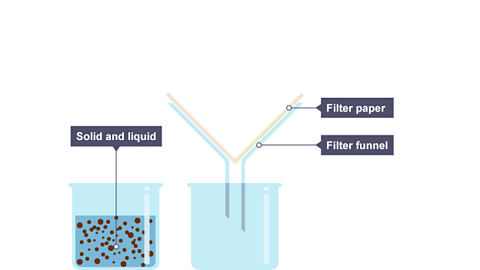
Image caption, A beaker containing a mixture of insoluble solid and liquid. There is filter paper in a filter funnel above another beaker.
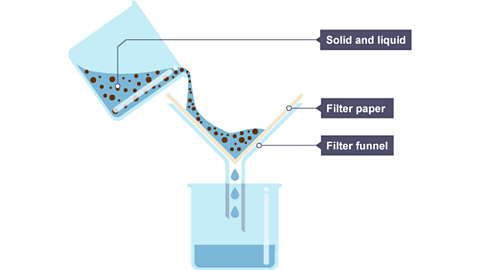
Image caption, The mixture of insoluble solid and liquid is poured into the filter funnel.
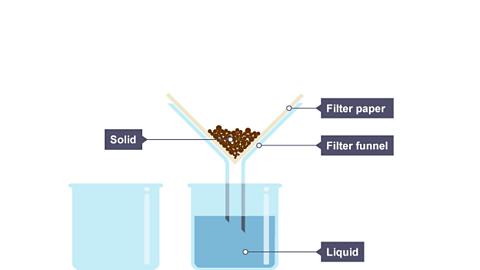
Image caption, The liquid particles are small enough to pass through the filter paper as a filtrate. The solid particles are too large to pass through the filter paper and stay behind as a residue.
1 of 3
NARRATOR: Solutions and filtration. Solutions are a mixture in which a solid or a gas has completely dissolved in a liquid.We call the solid a solute and the liquid a solvent. A solid that will not dissolve in a specific liquid is described as insoluble.For example, sand is insoluble in water.
We can separate insoluble solids from a solution using filtration.Filter paper has thousands of tiny holes that allow the liquid molecules to pass through.The filtered liquid is called the filtrate. The larger solid particles—in this case, the sand grains—are unable to pass through and are left on the paper. We call this the residue.
BEN: So, in front of me is a container of water and some children's play pit sand.
DR. TIM: Right, Ben. If you take that sand and pour it into the water. OK. And give it a good shake there, get it all mixed up.
BEN: OK, it's in there. I'll put the lid on. Make sure it clicks, so I'm not gonna spill it.OK, you ready?
DR. TIM: Ready. Right, now look at the result.
BEN: OK, here we go. I'll take the lid off. OK, there we go. Take a look at that. Now it's not a clear solution, is it?
DR. TIM: So, would we define the sand as insoluble in water?
BEN: That's exactly right, Ben. A substance is classed as insoluble if it does not fully dissolve in a specific solvent—in this case, the sand is insoluble in water.
DR. TIM: Now, why don't you try and filter out that sand from the water?
BEN: OK, some filtration. Sure thing. So I'm going to pour this carefully. I'm just going to try and get the sand. Right.
DR. TIM: Now, filtration is the process by which you remove an insoluble solid from the liquid. How's that going, Ben?
BEN: It's getting there. Just a little bit more, I think. OK, should we take a look? Now, here we go. I don't know if you can see, but the container now contains slightly clearer water at the bottom. And left in the filter paper here at the top, if I show you, is loads of the sand. Look at that, most of the grains are there and it's filtered through to the bottom.
DR. TIM: Fantastic. And in doing that filtration, you've highlighted two new terms that have their own chemistry definitions. So, the solids that are too large to pass through the filter paper—they're known as the residue.
BEN: OK. And the water in here is called?
DR.TIM: That's called the filtrate. And the filtrate is slightly clearer than before, though it is still a little bit cloudy. And that's because some of the smaller pieces of sand have managed to pass through the filter paper into the water.
BEN: I tell you what, Dr. Tim, you weren't lying when you said there were a lot of definitions. But I reckon they've all dissolved into my brain—having filtered out all of the unnecessary residue in there.
DR. TIM: Fantastic. Thank you so much.
Evaporation
Evaporation is used to separate a soluble solid (i.e. a solid that dissolves) from a liquid. For example, copper sulfate is soluble in water – its crystals dissolve in water to form copper sulfate solution.
During evaporation, the water evaporates away leaving solid copper sulfate crystals behind.
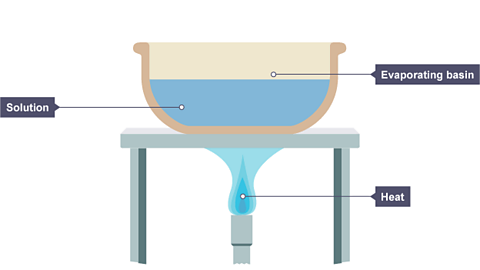
Image caption, A solution is placed in an evaporating basin and heated with a Bunsen burner.
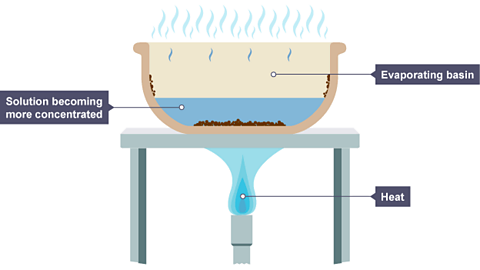
Image caption, The volume of the solution has decreased because some of the water has evaporated. Solid particles begin to form in the basin.
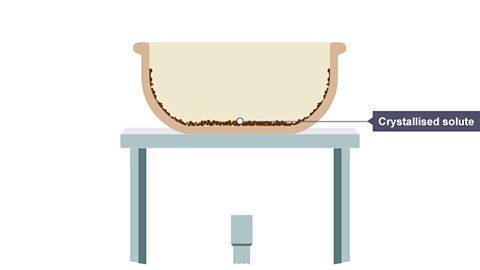
Image caption, All the water has evaporated, leaving solid crystals behind.
1 of 3
Simple distillation
Simple distillation is a method for separating the solvent from a solution. For example, water can be separated from salt solution by simple distillation.This method works because water has a much lower boiling point than salt. When the solution is heated, the water evaporates. It is then cooled and condensed into a separate container. The salt does not evaporate and so it stays behind.
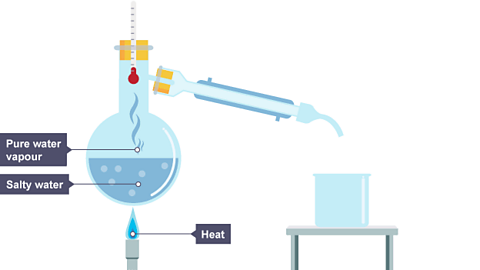
Image caption, Salt solution is heated.

Image caption, Water evaporates and its vapours rise. The water vapour passes into the condenser, where it cools and condenses. Liquid water drips into a beaker.
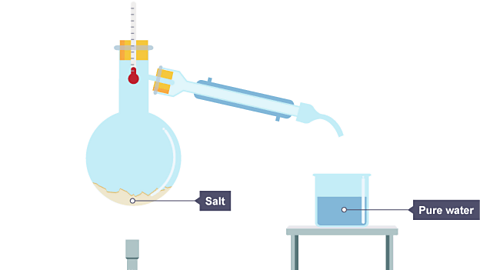
Image caption, All the water has evaporated from the salt solution, leaving the salt behind
1 of 3
Every pure substance has its own particular melting point and boiling point. One way to check the purity of the separated liquid is to measure its boiling point. For example, pure water boils at 100°C. If it contains any dissolved solids, like salt, its boiling point will be higher than 100°C. That is why salt is often added to water when cooking. The water boils at a higher temperature and so the food cooks more quickly.

Fractional distillation
Fractional distillation is a method for separating a liquid from a mixture of two or more liquids. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points.
When the mixture is heated, the liquid with the lowest boiling point boils first. The vapour condenses in the condenser before the other liquid boils. The long fractionating column ensures that the second liquid does not get into the condenser until most of the first one has been removed.
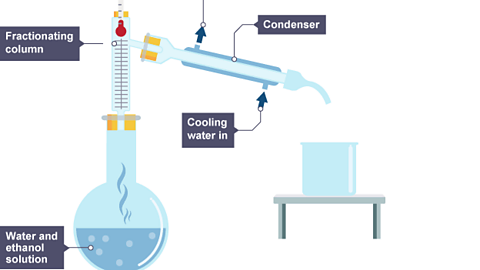
Image caption, A water and ethanol mixture is heated in a flask using an electric heater. Vapour forms in the air above the mixture in the flask
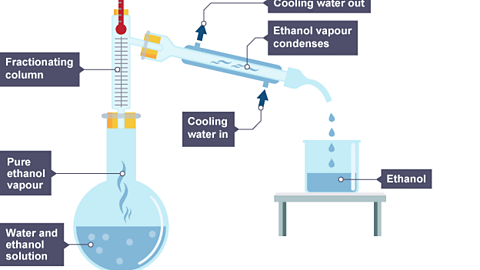
Image caption, The boiling point of ethanol is 78°C. Ethanol vapour passes into the condenser, where it is cooled and condensed. Liquid ethanol drips into a beaker.
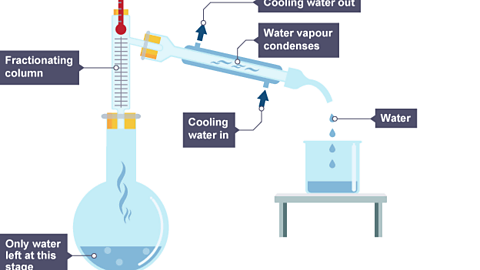
Image caption, When most of the ethanol has left, water vapour at 100°C passes into the condenser, where it is cooled and condensed. Liquid water now drips into a second beaker.
1 of 3
One way to check the purity of the separated liquids is to measure their boiling points. For example, pure ethanol boils at 78°C and pure water boils at 100°C.
Chromatography
Paper chromatography is a method for separating dissolved substances from one another. It is often used when the dissolved substances are coloured, such as inks, food colourings and plant dyes. For instance, ink is a solution of dyes dissolved in a solvent of water or oil.
It works because some of the coloured substances dissolve in the solvent better than others, so they travel further up the paper.
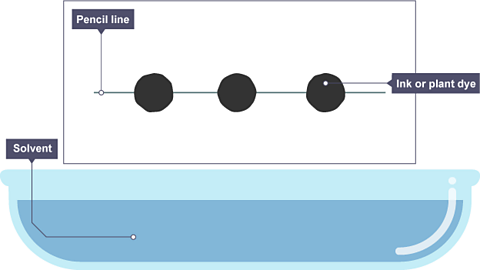
Image caption, A pencil line is drawn, and spots of ink or plant dye are placed on it. There is a container of solvent, such as water or ethanol.
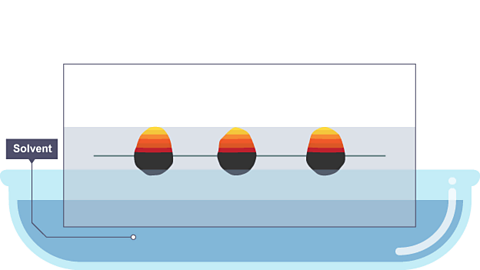
Image caption, The paper is lowered into the solvent. The solvent travels up through the paper, taking some of the coloured substances with it.
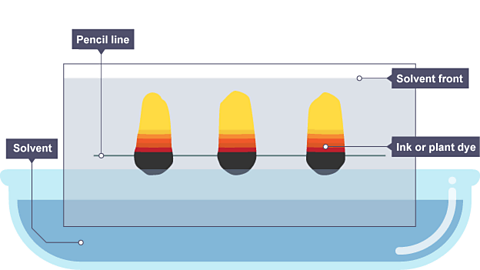
Image caption, As the solvent continues to travel up the paper, the different coloured substances spread apart. In this example, the separated coloured substances are yellow, red and black.
1 of 3
In this case the yellow substance is the most soluble as it travelled furthest up the paper.
A pure substance will only produce one spot on the chromatogram during paper chromatography.
Two substances will be the same if they produce the same colour of spot, and their spots travel the same distance up the paper.
In the example below, red, blue and yellow are three pure substances. The sample on the left is a mixture of all three.
Physical and chemical changes
Chemistry is about what matter is like and how substances behave and change. A substance can be changed by heating it, adding water to it, mixing it, cooling it, and so on. The change that takes place will be a chemical change or a physical change
Physical changes
Changes of state are examples of physical changes.No new substances are made, and the change is often easily reversed. For example:
- liquid water can be cooled down until it forms solid water (ice)
- ice can be heated until it forms liquid water again
- water vapour condenses to water
- iodine sublimises to purple vapour
Sugar dissolved in water is another example of a physical change. You can easily separate the two by distillation.
Chemical changes
Chemical changes are different from physical changes. New substances are made in chemical changes, and the change is often not easily reversed.
When a chemical reaction occurs, the change is called a chemical change. These include:
- methane burning in air, forming carbon dioxide and water
- iron becoming rust
This diagram shows what happens to iron and sulfur particles in a chemical reaction to form iron sulfide:
To begin with, iron particles are only joined to other iron particles, and sulfur particles are only joined to other sulfur particles. After the chemical change, iron particles and sulfur particles are joined to each other.
Chemical reactions
Atoms are rearranged in a chemical reaction. The substances that:
- react together are called the reactants
- are formed in the reaction are called the products
No atoms are created or destroyed in a chemical reaction. This means that the total mass of the reactants is the same as the total mass of the products. We say that mass is conserved in a chemical reaction.
Making iron sulfide
The reaction between iron and sulfur is often used to study elements and compounds. Iron sulfide is the compound produced in the reaction. The slideshow shows what happens in this reaction:
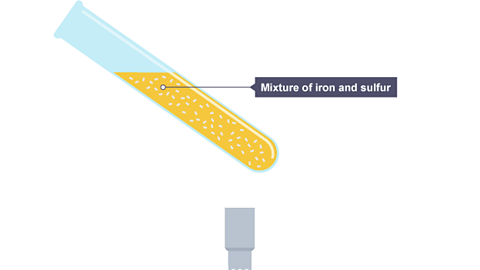
Image caption, The test tube is partly filled with a mixture of iron and sulfur
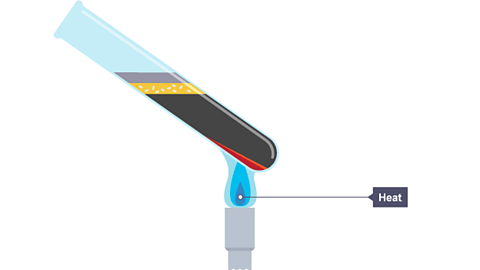
Image caption, The mixture is heated strongly using a Bunsen burner
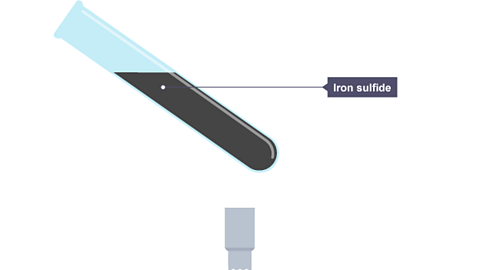
Image caption, The test tube now contains iron sulfide
1 of 3
Iron sulfide, the compound formed in the reaction, has different properties to the elements that it is made from. The table compares the properties of iron, sulfur and iron sulfide:
| Iron | Sulfur | Iron sulfide | |
|---|---|---|---|
| Type of substance | Element | Element | Compound |
| Colour | Silvery grey | Yellow | Black |
| Is it attracted to a magnet? | Yes | No | No |
| Reaction with hydrochloric acid | Hydrogen formed | No reaction | Hydrogen sulfide formed, which smells of rotten eggs |
The atoms in a compound are chemically joined together by strong forces called bonds. This is why the properties of a compound are different from the elements it contains, and why you can only separate its elements using another chemical reaction. Separation methods like filtration and distillation will not do this.
How to spot chemical change
A new chemical substance is formed which usually looks different from the starting substances e.g., set light to a mixture of oxygen, a colourless gas and hydrogen, a colourless gas and a chemical reaction occurs producing water. The new substance produced, water, is very different to both oxygen and hydrogen
Energy is taken in or given out e.g., when iron reacts with sulfur, the reaction gives out heat energy once it begins – it glows red hot
There is fizzing or sign of a new gas
There is a permanent colour change
The change is usually difficult to reverse

Summary
Physical change
- no new substances are made
- no chemical reaction occurs
- easily reversed
- e.g. change in state (solid, liquid, gas) like ice melting to water or making a mixture

Chemical change
- a new substance is always formed
- a chemical reaction occurs
- there may be fizzing or signs of a new gas
- heat or light produced
- there may be permanent colour change
- there may be loss of magnetism
- e.g., heating a mixture of iron and sulfur to produce iron sulfide

Chemical equations
The changes in chemical reactions can be expressed using equations.An equation is normally written in the form:
reactants → products
The reactants are shown on the left of the arrow, and the products are shown on the right of the arrow.Do not write an equals sign instead of an arrow. If there is more than one reactant or product, they are separated by a plus sign.
Word equations
A word equation shows the names of each substance involved in a reaction and does not include chemical symbols or formulae.For example:
copper + oxygen → copper oxide
The arrow means 'reacts to make'.In this reaction, copper and oxygen are the reactants, that react to make copper oxide, the product.
Balanced equationsA balanced equation gives more information about a chemical reaction because it includes the symbols and formulae of the substances involved. There are two steps in writing a balanced equation:
- replace the name of each substance with its symbol or formula
- use numbers to balance the equation, if it is not already balanced
In the example above (the reaction between copper and oxygen to make copper oxide), we get this in the first step:
Cu + \(O_2\) → CuO
This is unbalanced because there is one copper atom on each side of the arrow, but two oxygen atoms on the left and only one on the right.
To balance the equation, you need to adjust the number of units of some of the substances until we get equal numbers of each type of atom on both sides. You should never change the formula of a substance to do this.
Here is the balanced symbol equation:
2Cu + \(O_2\) → 2CuO
You can see that we now have two copper atoms and two oxygen atoms on each side. This matches what happens in the reaction:
When hydrogen and oxygen undergo a chemical reaction the product is water. The balanced equation is:
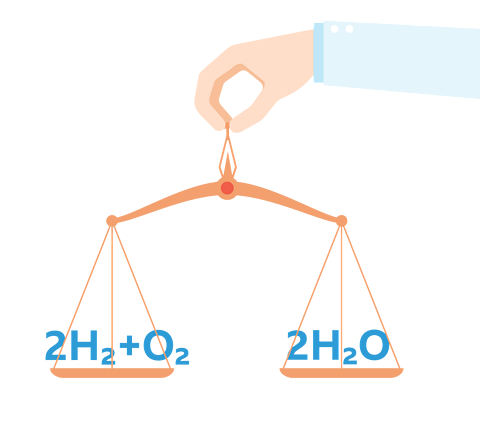
2\(H_2\) + \(O_2\) → 2\(H_2\)O
Two molecules of hydrogen and one molecule of oxygen react to produce two molecules of water.
The equation is balanced because:
- On the left there are four hydrogen atoms and two oxygen atoms
- On the right there are four hydrogen atoms and two oxygen atoms
Carbon and oxygen combine chemically to produce carbon dioxide
C + \(O_2\) → \(CO_2\)
One atom of carbon and one molecule of oxygen react to produce one molecule of carbon dioxide.
The equation is balanced because:
- On the left there is one carbon atom and two oxygen atoms
- On the right there is one carbon atom and two oxygen atoms
Here are some other examples of balanced equations. Check that you understand why they are balanced:
- 2Mg + \(O_2\) → 2MgO
- \(CuCO_3\) → CuO + \(CO_2\)
- Mg + 2HCl → \(MgCl_2\) + \(H_2\)

More word equations
Here are some more examples:
sodium + chlorine → sodium chloride
Notice: the non-metal (chlorine) changes its ending to ‘ide’
calcium + oxygen → calcium oxide
sodium + sulfur → sodium sulfide
magnesium + bromine → magnesium bromide
calcium carbonate → calcium oxide + carbon dioxide
Potassium hydroxide reacts with sulfuric acid. Potassium sulfate and water are formed in the reaction.
The word equation is:
potassium hydroxide + sulfuric acid → potassium sulfate + water
More on Chemistry
Find out more by working through a topic
- count2 of 8

- count3 of 8

- count4 of 8

- count5 of 8