Solids, liquids and gases
Solids, liquids and gases. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. This explains why solids have a fixed shape.
In a liquid like water, the particles are randomly arranged. They move freely over each other, a bit like marbles in a bucket.This is why liquids can be poured.
In a gas, like helium, particles are widely spaced apart and move quickly and random directions. They have no fixed volume and fill the space of their container. They travel in straight lines until they hit something, like the walls of the container they're in, or each other.
Three states of matter
Matter is the "stuff" that makes up the universe. Everything that has mass is matter.
In normal everyday life we come across matter in three states, solid, liquid and gas.
Solids
Steel, plastic and wood are solids at room temperature. Ice is solid water. The particles in a solid are:
- packed tightly together
- arranged in a regular way
Strong forces, called bonds, attract the particles towards each other. This means that the particles in a solid:
- can vibrate but are fixed in their position
- cannot move from place to place
- a force must be used to change the shape of a solid
The table shows some of the properties of solids and why they are like this:
| Property | Reason |
|---|---|
| They have a fixed shape and cannot flow | The particles cannot move from place to place |
| They cannot be compressed (squashed) | The particles are close together and have no space to move into |
| Have fixed volume | The particles cannot move from place to place |
Solids such as concrete are useful for buildings and their foundations because they cannot be compressed easily.
Liquids
Mercury, petrol and water are liquids at room temperature. The particles in a liquid are:
- close together, but slightly more loosely packed than solids
- arranged in a random way
The particles in a liquid can:
- flow around each other
The attractive forces (bonds) in a liquid are strong enough to keep the particles close together, but weak enough to let them move around each other.The table shows some of the properties of liquids and why they are like this:
| Property | Reason |
|---|---|
| They flow | The particles can move around each other |
| Take the shape of their container | The particles can move around each other |
| Have fixed volume | The particles remain in close contact with each other |
| They cannot be compressed (squashed) | The particles are close together and have no space to move into |
Liquids are useful in car brake systems because they flow and cannot be compressed easily
Gases
Oxygen, helium and chlorine are gases at room temperature. Water vapour is water as a gas. The particles in a gas are:
- far apart
- arranged in a random way
The particles in a gas can:
- move quickly in all directions
The attractive forces between the particles in a gas are very weak, so the particles are free to move in any direction.
The table shows some of the properties of gases and why they are like this:
| Property | Reason |
|---|---|
| They flow | The particles can move quickly in all directions |
| Completely fill their container | The particles can move in all directions and are not in close contact with each other |
| Have no fixed shape or volume | The particles spread out in all directions and fill any container |
| They can be compressed (squashed) | The particles are far apart and have space to move into |
Gas pressure
The particles in a gas move quickly in all directions, but they do not get far before they bump into each other or the walls of their container.Gas pressure is caused by the gas particles hitting the walls of their container. If the temperature is increased, the particles in a gas move faster, and they hit the walls of the container more often. This causes the pressure to rise.The pressure of a gas will also increase when the volume of its container is decreased. Once again, the particles in a gas hit the walls of the container more often, causing the pressure to rise.

Change of state
Most substances can exist as a solid, liquid or gas. They can change state, usually when they are heated or cooled. For example, liquid water turns into water vapour when it is heated enough, and it turns into ice when it is cooled enough.The closeness, arrangement and motion of the particles in a substance change when it changes state. Simple diagrams of particles in a solid, liquid and a gas are shown like this:
See how energy is involved in a substance's changing state.
Energy in changes of state.
Substances can change states. From solid to liquid to gas and back again. And this usually happens when they are heated or cooled.
It's Keisha’s birthday and her dad has baked a cake and wants to cover it with chocolate. He puts a solid chocolate bar in a bowl and warms it over hot water. As the chocolate heats up it begins to melt. The chocolate is absorbing energy from its surroundings. The heat from the water below. This is known as an endothermic process, where heat is absorbed during the change of state.
The chocolate is now a liquid and Keisha’s dad is able to pour it over the cake. After a few hours, Keisha notices that the chocolate is now solid, its state has changed back again. This is an exothermic process. The heat energy that the chocolate absorbed is released, causing it to cool down and become solid.
Keisha’s dad lights her birthday candles. Better blow them out before the candles melt.
Gaining energy
The table summarises what happens to the particles in a substance when it gains energy, and it melts or evaporates i.e., changes state:
| Melting | Evaporating/Boiling | |
|---|---|---|
| Description | Solid to liquid | Liquid to gas |
| Closeness of particles | Stay close together | Become much further apart |
| Arrangement of particles | Regular to random | Stay random |
| Motion of particles | Start to move around each other | Move quickly in all directions |
Evaporation happens below the boiling point of a liquid and only occurs from the topmost layer of the liquid.When the liquid reaches its boiling point, evaporation happens very quickly and from the entire liquid. That is why it boils.
Losing energy
The table summarises what happens to the particles in a substance when it loses energy, and it freezes or condenses i.e. changes state like ice - water - steam:
| Condensing | Freezing | |
|---|---|---|
| Description | Gas to liquid | Liquid to solid |
| Closeness of particles | Become much closer together | Stay close together |
| Arrangement of particles | Stay random | Random to regular |
| Motion of particles | Stop moving quickly in all directions, and can only move around each other | Stop moving around each other, and only vibrate about a fixed position |
Models for atomic structure for solid, liquid and gas
The diagram summarises these changes of state:
Sublimation
Some substances can change directly from solid to gas, or from gas to solid, without going through the liquid stage. This is called sublimation. Solid carbon dioxide ('dry ice') and iodine can sublime.
Conservation of mass
The particles in a substance stay the same when it changes state - only their closeness, arrangement and speed change. This means that the mass of the substance stays the same. For example, 10 g of water boils to form 10 g of steam or freezes to form 10 g of ice. There is change of state, but no change of mass. This is called conservation of mass.
Miss Roberts: So, today we're looking at the conservation of mass.
Katie: Oh, the conservation of mass. Where do we start with this one?
Miss Roberts: It sounds tricky, but it's easier if I just show you.
Katie: OK, great. Straight in with an experiment. This is my kind of science.
Miss Roberts: So I want you to put that bottle of water, please, on to the scales and tell me what it says in grams.
Katie: OK, it's says three hundred grams.
Miss Roberts: OK, so we are looking at the conservation of mass. Now, mass is the measure of how much stuff or matter is in an object and it's measured in either grams or kilograms.
Katie: Right, OK. So, this bottle of water has a mass of three hundred grams.
Miss Roberts: It does. And I want to show you what happens when a chemical reaction takes place or when there is a physical change in something that the mass stays the same. It is what's called 'conserved'.
Katie: OK. So, what's with the bottle of water, then? Are you just thirsty?
Miss Roberts: So, when you freeze water a physical change happens, because it turns from water to ice. But, the interesting thing is that although the water changes to ice the mass will stay the same. So, earlier I froze an identical bottle with three hundred grams of water. What do you see?
Katie: Well, what I see there is that it's increased. You know, the ice fills up more of the bottle. So, surely the mass has increased, proving science wrong?
Miss Roberts: Well, we'll see.
Katie: OK. Let's weigh it shall we?
Three hundred grams. It's exactly the same.
Miss Roberts: So, it may look like the mass has increased, because the ice takes up more room than the liquid water. But, the mass has in fact stayed the same.
Katie: What is this? Sorcery?
Miss Roberts: No, it's because the tiny particles that make up stuff or the matter in an object - known as atoms - they cannot be destroyed. So, when a physical change or a reaction takes place, atoms in a starting substance rearrange themselves and join back together to form a new substance. This is what we call a 'product'. But, the number of atoms will still be the same before and after reaction. So the total mass is the same.
Katie: That's amazing. So, when water freezes it makes the atoms rearrange themselves so the result is the ice. But, because atoms can't be destroyed, the ice still has the same amount of stuff in. It just looks completely different.
Miss Roberts: Exactly. So, in other words, the mass is conserved.
Katie: Wow. That's amazing. That's blown my mind, Miss Roberts. It really has. Here's a couple more examples to prove it too.
VOICEOVER: Zach has a bowl of ice cubes with a mass of five hundred grams. After some time the ice cubes melt, but the scales still show five hundred grams. This is because melting the ice has not created or destroyed any atoms and the mass has stayed the same. This is known as the law of conservation of mass. It applies to both chemical reactions and physical changes.
Zach puts a log on the fire. When it burns, Zac wonders if the ashes will be the same mass as the log was before. During burning, oxygen reacts with the log and releases carbon dioxide and water vapour - leaving solid ash. So, the mass of the ash is less than the loch. But, if we measured the mass of the gases produced and the ash, the total would be equal to the mass of the log and the oxygen used up during the combustion. This is because the mass of all the atoms will always stay the same.
Kinetic theory
Kinetic theory explains that matter is made up of tiny particles which constantly move. Forces hold the particles together which vary in size for solids, liquids and gases. This is called kinetic theory.
Atoms and molecules
The simplest particles of matter are called atoms. Everything is made from atoms, including you.
Atoms are tiny particles that are far too small to see, even with the most powerful microscope.If people were the same size as atoms, the entire population of the world would fit into a box about a thousandth of a millimetre across!
We usually imagine atoms as being like tiny balls:

To make diagrams simpler we often draw atoms as circles:
A piece of pure gold contains only gold atoms. A piece of pure lead contains only lead atoms.
Molecules
Sometimes two or more atoms join together. This forms a molecule. The atoms that form the molecule are held together by chemical bonds.
Hydrogen molecules – made from two hydrogen atoms bound together
Oxygen molecules - made from two oxygen atoms bound together
Test your knowledge
Diffusion
How does the movement of particles and gases cause diffusion?
Gethin : OK, Miss Armit, what exactly is the particle model.
Miss Armit: The particle model as a way of explaining the way particles move and collide. There's four things you need to know about it. All substances are made of particles. The particles are attracted to each other - some more than others. And the particles are constantly moving with what's called 'Kinetic Energy'. As temperature increases, this kinetic energy increases so they can move around more.
Gethin : OK. Those are the four things to remember. Now, substances are made up of solids, liquids and gases. Do the particles move around in the same way in each?
Miss Armit: No, not quite. They move round differently in each one. So in gases they are far freer to move around than in liquids and in solids. They have a lower density and they're far apart. In solids they're much closer together and they can only vibrate. And liquids are kind of in between. Their particles are spread out randomly and their density is less than the solids, but higher than gases.
VOICEOVER: The particles that make up gases and liquids move randomly. This movement allows the action of diffusion to happen.
For example, if you spray some deodorant in your bedroom. The particles move from an area of high concentration, the place where you sprayed it, to an area of lower concentration - your bedroom doorway. Then, through the rest of the house. The perfume particles have diffused.
Gethin : I got all excited because I thought Miss Armit brought in sweets and balloons for a bit of a party. But that's not the case, it's actually for an experiment. But, how and why have you brought them in? And what's it got to do with the particle model?
Miss Armit: Well, articles are too small to see. So in order to demonstrate the model we use these to help demonstrate it, so we can see them in different states of matter.
Gethin : What about diffusion? Can happen in all states? And by that I mean solids, liquids and gases?
Miss Armit: So, it happens mainly liquids and gases. This is because the particles can freely move around. It doesn't really happen in solids as the particles are in this fixed position and they can only vibrate on the spot.
Gethin : Alright. And have you got an example of that in front of you, then?
Miss Armit: Yeah, just here. I poured some water on this plate earlier. And this is before and after. So, what happened is the food colouring on the outside of the sweets has dissolved into the water. And then it's moved from the high concentration of food colouring around the sweet to the lower concentration in the middle. And you can clearly see the diffusion is illustrated.
You walk into a coffee shop and immediately smell coffee. Why?
One reason is moving air currents or convection. Moving currents of air carry coffee smell particles all around the coffee shop.
But even without convection, the smell would still reach you eventually - by diffusion. In diffusion, coffee particles move from the coffee machine (an area of high concentration) to the rest of the coffee shop (an area of low concentration).
Diffusion is the particles of one substance spreading out and mixing with the particles of another substance.
Making a cup of coffee also involves the diffusion of coffee particles through hot water:
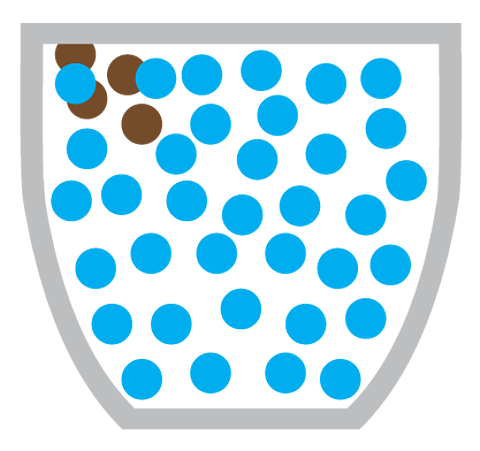
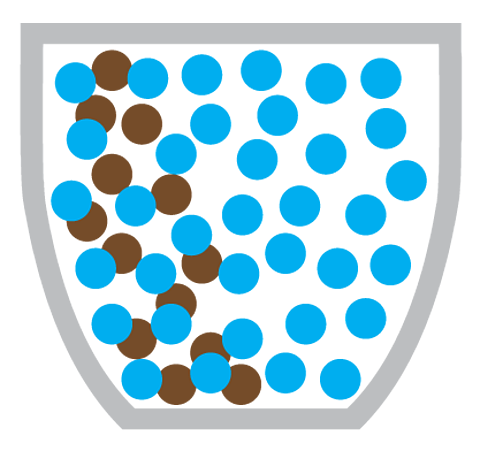
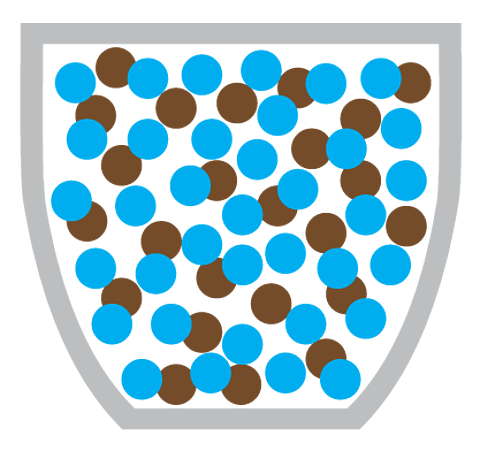
What causes diffusion?
In gases and liquids, particles move randomly from place to place. The particles collide with each other or with their container. This makes them change direction. Eventually, the particles are spread through the whole liquid or whole container.
Diffusion happens on its own, without stirring, shaking or wafting.
Why is diffusion useful?
In living things, substances move in and out of cells by diffusion. For example:
Respiration produces waste carbon dioxide, causing the amount of carbon dioxide to increase in the cell. Eventually, the carbon dioxide concentration in the cell is higher than that in the surrounding blood. The carbon dioxide then diffuses out through the cell membrane and into the blood.
Water diffuses into plants through their root hair cells. The water moves from an area of high concentration (in the soil) to an area of lower concentration (in the root hair cell). This is because root hair cells are partially permeable. The diffusion of water like this, is called osmosis.
Diffusion in gases
Diffusion occurs because of differences in concentration. When chemical substances such as perfume are let loose in a room, their particles mix with the particles of air.
The particles are free to move quickly in all directions. They eventually spread through the whole room from an area of high concentration to an area of low concentration. This is called the diffusion gradient.
Diffusion always occurs in the same direction:
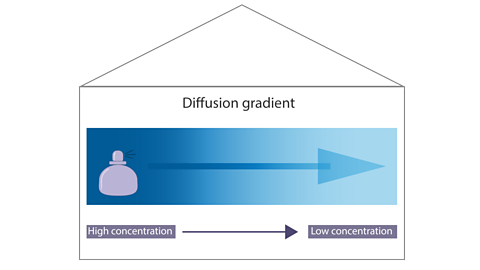
The process continues until the concentration of the particles of smelly gas is the same throughout the room. Remember that the particles still move, even when the smell is evenly spread.
You do not have to mix the gases by waving your arms around - they mix on their own.
Diffusion in gases is quick because the particles in a gas move quickly. It happens even faster in hot gases because the particles of gas move faster.
Diffusion of gases: ammonia and hydrogen chloride.
This experiment shows how particles diffuse in vapour at different speeds.
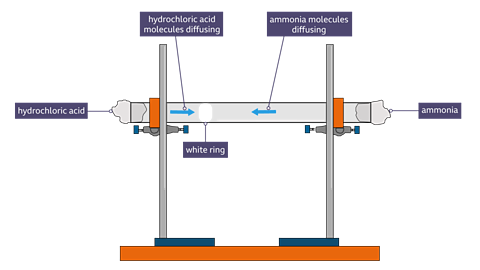
Equipment:
- Glass tube
- Concentrated hydrochloric acid
- Concentrated ammonia solution
- Cotton wool and glass Pasteur pipettes
The glass tube was fixed horizontally using a standard laboratory clamp and stand.Concentrated hydrochloric acid was dropped onto some cotton wool at one end of the tube and some concentrated ammonia was added to some cotton wool at the other end.
After a couple of minutes, a white ring of ammonium chloride ( a solid) will form where the two gases meet. This will be closer to the source of the hydrogen chloride than to the source of the ammonia because hydrogen chloride diffuses more slowly than ammonia.
Brownian motion
Gas particles move very quickly - particles in air move at 500 m/s on average at room temperature.
However, a smell does not travel this fast. This is because its particles collide with each other and with particles of air very frequently. They change direction randomly when they collide, so it takes much longer to travel from one place to another. Their random motion because of collisions is called Brownian motion.
Diffusion in liquids
Diffusion can also happen in liquids. This is because the particles in liquids can move around each other, which means that eventually they are evenly mixed.
For example, potassium manganate(VII) is a purple solid. If you put a crystal of it into a jar of water, the purple colour spreads slowly through the water. This is by diffusion. The slideshow describes what happens.
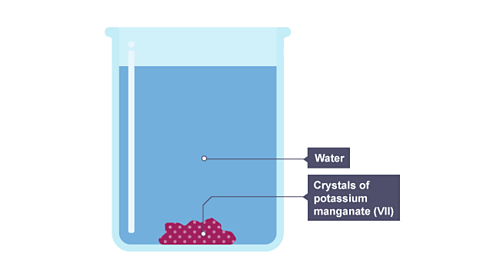
Image caption, The purple colour begins to spread from the crystal
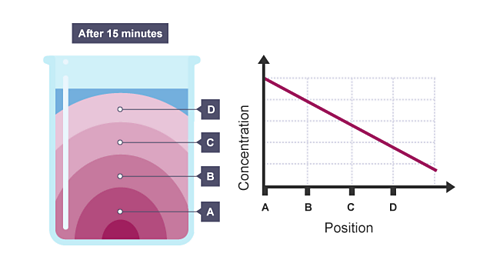
Image caption, The colour gradually spreads through the water as diffusion happens
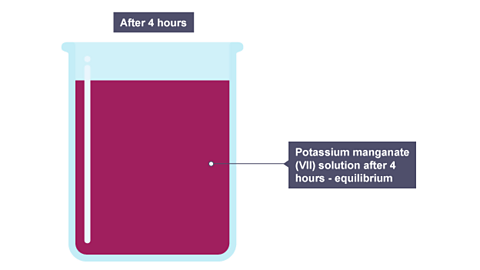
Image caption, After a few hours, diffusion has produced a potassium manganate(VII) solution where the concentration is the same everywhere in the solution
1 of 3
After a few hours, diffusion has produced a potassium manganate(VII) solution where the concentration is the same everywhere in the solution.
Diffusion in liquids is slower than diffusion in gases because the particles of a liquid move more slowly than the particles of a gas.
Expansion and contraction
Kinetic theory can also be used to explain expansion and contraction.
Substances expand (increase in size) when they get warmer, and they contract (decrease in size) when they get cooler. This property can be useful. For example:
- Thermometers work because the liquid inside them expands and rises up the tube when it gets hotter.
- Metal parts can be fitted together without welding, using shrink fitting. The slideshow shows how this works:
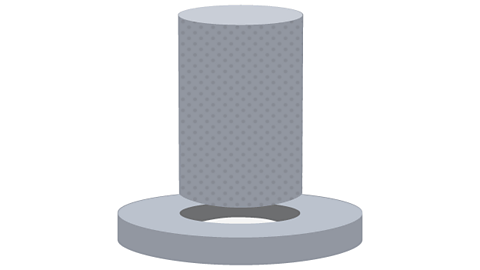
Image caption, The rod is too large to fit through the hole

Image caption, The rod is cooled down, making it contract enough to fit through the hole
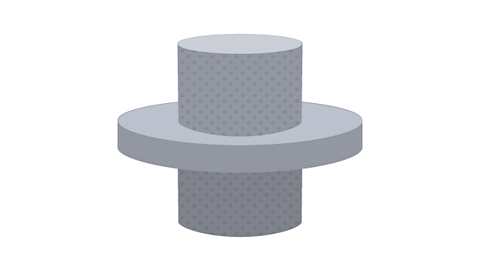
Image caption, The rod is allowed to warm back up. It expands and fits tightly
1 of 3

The expansion and contraction of materials can also cause problems. For example, bridges expand in the summer heat and need special joints to stop them bending out of shape.

What do the particles do?
When substances expand or contract, their particles stay the same size. It is the space between the particles that changes:
- the particles in a solid vibrate faster when it is heated, and take up more room – volume increases
- the particles in a liquid move around each other faster when it is heated, and take up more room– volume increases
- the particles in a gas move faster in all directions when it is heated, and take up more room– volume increases
The particles of a liquid and gas move more freely than the particles of a solid, so they contract and expand more than solids.
Demonstration of solid expansion
Ball and ring
If the ball fits through the ring at room temperature, heat the metal ball. It expands and will then not fit through the ring.
If the ball does not fit through the ring at room temperature heat the metal ring.The ring expands and the hole in the centre gets bigger! The ball will now fit through the ring.
Demonstration of liquid expansion
Liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight, liquids expand more than solids.
The water level in the capillary tube is initially at A.
When the water is heated the liquid level initially falls to level B, before rising to level C and above.
When the glass flask and capillary tube are first heated, they expand. This causes the volume of the flask to increases, and the liquid level falls to B.
However, the liquid is quickly heated and starts to expand. It expands more than the solid glass and so the water level rises back to A and then up to, and beyond, C.
This shows that:
- Liquids expand when heated
- Liquids expand more than solids
This is the principle behind liquid-in-glass thermometers. An increase in temperature results in the expansion of the liquid which means it rises up the glass. The liquid used is usually alcohol or mercury.
Demonstration that gases expand
Gases also expand when heated
Molecules within gases are further apart and weakly attracted to each other. Heat causes the molecules to move faster, which means that the volume of a gas increases more than the volume of a solid or liquid.
When you stop heating the flask it cools and contracts. This causes the liquid to get sucked up the tube and into the flask!
Summary
- Matter expands when heated and contracts when cooled
- Liquids expand and contract more than solids
- Gases expand and contract more than liquids
The bimetallic strip
A bimetallic strip is made from two different metals riveted together, one on top of the other.
When heated, both metals expand, but one expands more than the other. Since they are riveted together, they cannot slip over one another, and the strip bends.
The metal which expands more is at the outside of the curve. The metals used are often brass and steel. Brass expands more than steel and so will be on the outside of the curve.
As the metal cools it straightens out again. It bends in the opposite direction if cooled down beneath room temperature.
A bimetallic fire alarm
When there is a fire:
- The temperature rises
- The brass expands more than the iron and will be on the outside of the curve
- The strip bends down and touches the contact
- The electric circuit is complete and the bell rings
Getting temperatures right isn't just something you need to do with living things.
Now these two bars are made of two different metals - brass and iron.
Now if I heat them in the same flame, brass expands more than the iron.
So if I fix them together, and then heat them in the same flame, what do you think will happen?
Both still expand but by different amounts and the force generated is big enough to bend the bars and in some jobs knowing this can be very important indeed.
Density of solids, liquids and gases
The difference between the densities of solids, liquids and gases is due to the distance between the particles in each state of matter.
Solids and liquids
The particles of a solid are very close together.It melts when it changes from the solid state to the liquid state. The particles of a liquid remain close together, so there is usually only a small increase of volume.So, the same mass of liquid will have slightly greater volume than the solid.
As density = \(\frac{mass}{volume}\), the liquid will have slightly lower density.
The density of solid iron = 7.8 g/cm³The density of liquid iron = 6.9 g/cm³
Liquids and gases
A substance evaporates when it changes from the liquid state to the gas state. Its particles move freely and are very far apart, so there is a large increase of volume.The same mass of gas will have very much greater volume than the liquid, and so will have much lower density.The density of liquid oxygen = 1.1 g/cm³The density of gaseous oxygen = 0.0014 g/cm³
| State | Distance between particles | Density | Density in g/cm³ |
|---|---|---|---|
| Solid | Very close together | High | Solid iron = 7.8 |
| Liquid | Slightly further apart than a solid | Slightly less than the solid | Liquid iron = 6.9 |
| Gas | Very much further apart than a solid or liquid | Very much less than the solid or liquid | Oxygen gas = 0.0014 |
More on Chemistry
Find out more by working through a topic
- count3 of 8

- count4 of 8

- count5 of 8
- count6 of 8
