Key points
- atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons. are the building blocks of everything.
- Atoms can form strong bonds with each other, making moleculeTwo or more atoms which are strongly bonded together. The smallest particle of a substance that has all of the physical and chemical properties of that substance..
- A pure substance made from only one type of atom is called an element. Elements are listed on the periodic table.
- Elements can combine to make compounds.
Why is it difficult to see an individual atom?
It is difficult to see an individual atom because they are so small. We use microscopes to see tiny things like bacteria but these are about 10,000 times bigger than an atom.
Atoms and molecules
Watch this video to find out more about atoms and molecules.
Teacher: What do you think you're made of?
Presenter: [SIGHS]
Big question. Grit, determination, hardworking, handsome, charming, funny.
Teacher: Well, I guess. But you're actually made of atoms. In fact, everything is made of atoms.
Presenter: It's funny, because I don't remember seeing any atoms last time I looked in the mirror. Just these handsomely, devilish good looks.
Teacher: Well, atoms are the tiny particles that are too small to see, even with a microscope.
So if people were the size of an atom, the entire population of the world would fit into a box about 1000th of a millimetre across.
Presenter: Wow, teeny tiny. Well, is there a way to help us visualise atoms to help us learn about them?
Teacher: Well, we usually imagine atoms to be like tiny balls like this. Now, to make the diagrams simpler we often just draw them as circles. So now an atom is the smallest particle of an element. There are over one hundred different elements and these are all made of atoms.
Presenter: OK. Well, before we get into this further, let's check out the top things to know about all of this.
VO: Particle diagrams.
An atom is the simplest particle of an element. In a particle diagram, atoms are represented by a single coloured circle. When atoms of two or more elements bond together, they make a compound. These clusters of strongly bonded atoms are called molecules. See how they're represented by two circles of different size and colour that are joined together. This particle diagram represents water or, to use its chemical formula, H₂O which is a compound of the elements hydrogen and oxygen.
In one molecule of water, there are two hydrogen atoms and one oxygen atom. Particle diagrams can also show the ratio of atoms of each element in a compound which in turn can tell us the formula of the compound - H₂O.
Presenter: OK, let's make sense of all this with Mrs Roberts. Are elements made up of a mixture of different types of atoms?
Teacher: No, elements are made of only one type of atom. So, different elements are made of different atoms. For example, we've got lead and we've got gold. They're both elements.
Presenter: Mm-hm.
Teacher: But a piece of pure gold will contain only gold atoms, whereas a piece of pure lead will contain only lead atoms.
Presenter: I get it, right. And then can we mix different atoms together?
Teacher: Yeah. So, two or more elements that chemically bond together will form a compound.
Presenter: Ah, right. OK, so, can you give us some examples of compounds? Could I find some at home?
Teacher: Absolutely. We're surrounded by lots and lots of different compounds all of the time. For example, we've got water. Water is a compound of hydrogen and oxygen. The chemical name for water is H₂O because each of its molecules contain two hydrogen atoms and one oxygen atom.
Presenter: So we call these molecules?
Teacher: Yes. So, a molecule is a word we use to represent the simplest unit of some compounds. These little red blobs represent the oxygen atoms. And then the two white circles, they represent the two hydrogen atoms that are chemically bonded together.
Presenter: So all of these molecules together make water, which is a compound.
Teacher: Correct.
What is the difference between an atom and a molecule?
An atom is the smallest particle of an element. A molecule is made of two or more atoms chemically bonded together.
Atoms
Atoms are the building blocks of all matter. Everything is made of atoms - even yourself.
Atoms are the smallest particle of an elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table., which are far too small to see. Even the most powerful microscopes cannot visualise a single atom. If the earth’s population of 7 billion people were the size of an atom, they would take up less than 1mm of space.

In science, the word particleA single piece of matter from an element or a compound, which is too small to be seen. Particles can be atoms, molecules or ions. can be used in many ways. It is a single piece of matter from an element or a compound, which is too small to be seen. Particles can be atoms, molecules or ions.
Elements
An element is a pure substance which is made from only one type of atom. Everything in the universe contains the atoms of one or more elements. The atoms in one element are all the same as each other, but they are different from the atoms of any other elements. There are 118 different elements. They are listed on the periodic tableA table which lists all of the chemical elements and arranges them in a way that is useful. It allows us to spot patterns and make predictions about other elements.. Examples of elements include oxygen, hydrogen and carbon.
Gold is an element. Which atoms are pure gold bars made of?

In pure gold bars, all of the atoms are gold.
Molecules
Molecules are made when two or more atoms chemically bond together. Atoms from different elements can combine. When the atoms are from different elements, the molecule can also be called a compound.
The atoms of some elements like helium do not form bonds. Helium atoms are single atoms.
The atoms of elements such as hydrogen, nitrogen and oxygen join in pairs to make molecules.
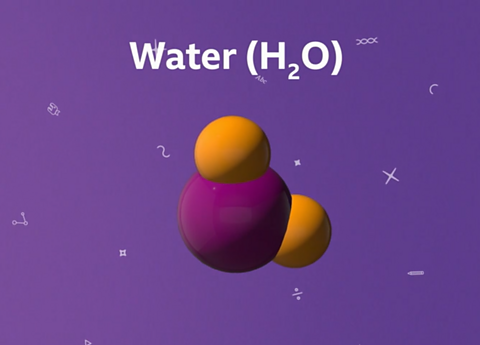
Water is made of molecules. Each water molecule is made from two hydrogen atoms chemically bonded to one oxygen atom. This means that the chemical formula of water is H₂O.
Carbon dioxide is made of molecules of a carbon atom bonded to two oxygen atoms (CO₂).

Drawing atoms and molecules
Atoms can be drawn as circles. But all the atoms of an element need to look exactly the same.
When drawing a diagram of molecules made from the atoms of more than one element, different sizes and colours can show the different elements.

There are no rules for the colours of the atoms when drawing them.
They can be any colour.
Examples
Water (H₂O) molecules contain one oxygen atom and two hydrogen atoms.
Here, the oxygen atom is red and the hydrogen atoms are white.
Ethanol is a much larger molecule made of three types of atoms. Its formula is CH₃CH₂OH.
Here, the oxygen atom is red, carbon is black and hydrogen is white.
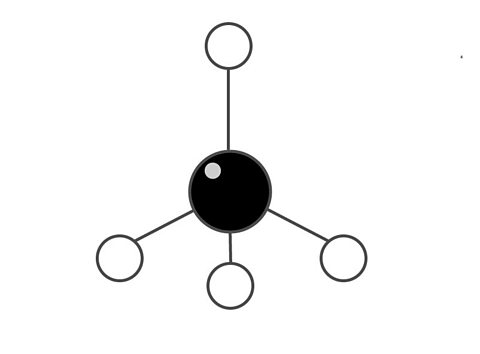
Molecules can be drawn in different ways. In this diagram of methane (CH₄) the chemical bond between atoms is shown as a stick.
One molecule of the compound methane is made from one carbon atom and four hydrogen atoms.
These are carbon dioxide molecules. Can you work out what elements the different coloured circles are?
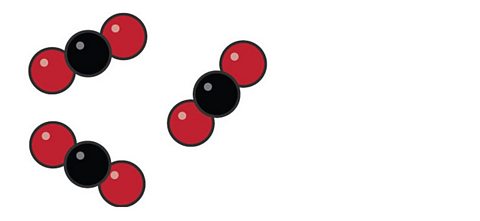
The black circles are carbon atoms and the red ones are oxygen. Carbon dioxide molecules contain one carbon atom and two oxygen atoms. The formula for carbon dioxide is written as CO₂.
Single element molecules
- Some molecules are made from one type of atom. This means the molecule is an element.
- Oxygen is a non-metal element and is found naturally as a molecule. Each one is made up of two oxygen atoms that are strongly bonded together.
- At room temperature oxygen is a gas.
An oxygen molecule always exists as a pair of atoms.
Test your knowledge
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on Atoms, elements and compounds
Find out more by working through a topic
- count3 of 8
- count4 of 8

- count5 of 8

- count6 of 8
