| |  |  |  |  |  |  | Home | About Atoms | The Periodic Table |  |  |  |  |  |  |  |  |  |  |    |  |  |  |  |  |  |  |  |  |  |  |  | | There are more than a hundred different elements in the universe. And, of course, arranging them in some kind of order would help! They could be arranged in alphabetical order but that wouldn't tell you much about their chemistry. In the periodic table, the elements are arranged according to their atomic number. | | Notice how the atomic number (top left hand corner) increases as you move across the table. Normally, the higher the atomic number, the heavier the element. So lead (Pb), atomic number 82 is heavier than tin (Sn) atomic number 50. Examine the periodic table and find out which is heavier gold (Au) or silver (Ag)? |
| |  |  |  | 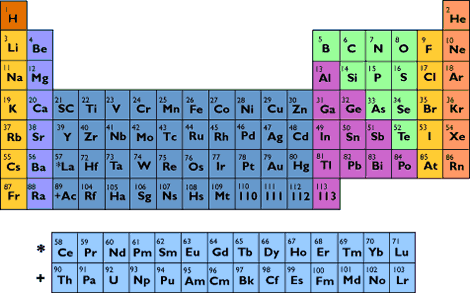 |  |  | | The behaviour of different elements changes periodically as their atomic number increases. In other words, if you know the atomic number of an element, then you can predict how it's likely to behave, in the same way that you can predict the behaviour of the Moon. The shape of the moon (as seen from earth) changes periodically, according to the day. In other words, if we know what day it is, we can predict the shape of the moon. Every 28th day, there is a full moon. | | Now try reading the periodic table up and down, not left to right... | | The elements are arranged in columns, or groups, so that the periodic behaviour of the elements can be seen at a glance. Each group of elements behaves in a similar fashion. | | Take Group One, for example. All the elements in group one (on the far left hand side) are metals. They are all highly reactive and they all form alkali solutions in water. This is why they are sometimes called the alkali metals. Sodium (Na) is the most common Group One metal. | | The Russian scientist, Dmitri Mendeleev, who compiled the first periodic table, left spaces for elements which he thought ought to exist but which had not yet been discovered. And, sure enough, just over a hundred years after Mendeleev designed his periodic table, all these spaces have been filled! |
| | |  |  |  |  | |  |
|



