The density of an object or substance is its mass divided by its volume: Density = Mass ÷ Volume.
The units of density depend on the units used for mass and volume, but are usually: g/cm³ (if mass is measured in g and volume in cm³).
The more dense a substance is, the heavier it feels for its size.
Narrator: I was quite surprised to find out how much maths is used in underwater filming.
Calculating density, calculating how long you can stay underwater.
When we go diving we have to think about a lot of things: Pressure, buoyancy, and particularly density.
For example, the density of fresh, clean water is approximately 1g per cubic centimetre.
Something like a cork, which is less dense than water, floats.
Something like a stone, which is more dense than water, sinks.
And as a diver, you neither want to float like a cork nor sink into the depths like a stone.
Water has a consistent density, but the deeper you go, the more pressure there is.
When we’re diving in cold water, we wear a dry suit,
and that traps a layer of air in dry clothes to keep us warm.
So we add air to the suit the deeper we dive,
to take away the squeeze, but more importantly,
it’s to help us become the same density as the water around us.

Density values
The densities of some everyday substances are:
Steel has a density of 7.82 g/cm³
Water has a density of 1.00 g/cm³
Air has a density of 0.0013 g/cm³
These values show that the steel (solid) is the most dense while the air (gas) is the least dense.

Density values
The densities of some everyday substances are:
Steel has a density of 7.82 g/cm³
Water has a density of 1.00 g/cm³
Air has a density of 0.0013 g/cm³
These values show that the steel (solid) is the most dense while the air (gas) is the least dense.
More on Compound measures
Find out more by working through a topic
- count5 of 5

- count1 of 5
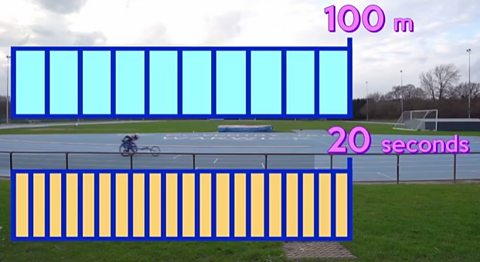
- count2 of 5
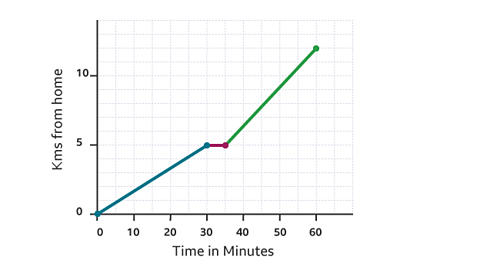
- count3 of 5
