Dr Yan - whipped Marmite and clouds
Yan looks at white Marmite

At one of the Roadshows, a man asked me why Marmite went white when stirred repeatedly. I hadn't heard of this before, but it's a fantastic effect, and easy to try yourself at home - if you're patient. By the way, I tried this with other similar products, but couldn't get them to work so well.
What's going on?
So how can you make something so dark become so white? I took some pictures through a microscope to investigate.
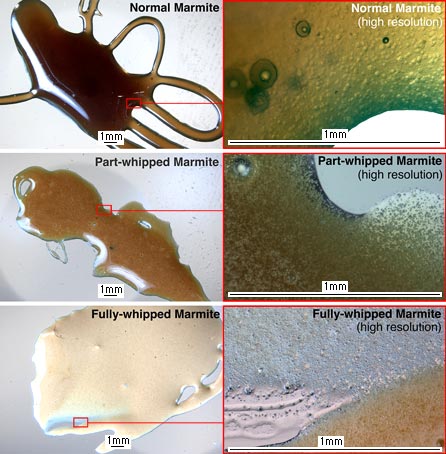
The first one is normal-looking Marmite. You can see that some light gets reflected, such as the mirror-like reflection of the fluorescent light on the left side of the Marmite blob. But once light has entered the Marmite it has a good chance of being absorbed, making the Marmite look dark. The thicker the Marmite, the more absorption takes place. For example, the thick middle of the blob is very dark, but the loops around the outside are thin enough to allow some of the background light to come though, so are pale brown. The detailed photo of the edge of the normal Marmite shows this same brownness (and also reveals that normal Marmite contains small blobs of translucent material about a fiftieth of a millimetre in diameter).
Marmite magnified
The other photos show the colour change when whipping Marmite. The detailed versions show what's going on: it becomes full of tiny bubbles. The more you stir, the more bubbles are crammed in, and the whiter it looks. Zoom in on the bubbles and you can see that they range in size but are generally less than less than a hundredth of a millimetre in diameter, regardless of whether the Marmite has been intensely whipped, half-whipped, or even just stroked repeatedly. Of course, whisking most liquids will fill them full of bubbles, but Marmite is so sticky and viscous that the bubbles stay in it for a long time. Nevertheless, they do eventually float to the surface and pop. You can test this by leaving your whisked marmite to settle for a couple of days – it should go dark again.
Fizzy drink foam
Lots of bubbly foams look quite white, regardless of the colour of the original liquid. Think, for example, of coloured fizzy drinks (eg cola foam), washing-up liquid, and heads on pints of beer. The reason for this whiteness is fascinating, and it involves the process that occurs as light travels from one environment (eg Marmite) to another (eg the air in a bubble). What happens is that the particles of light can get scattered, resulting in them changing direction. Some scattering alters the path of the light in a predetermined way, like the reflection that you get in a mirror, or the bending effect known as 'refraction' that you get when looking at objects through water or glass. Other sorts of scattering are more random, such as deflection off tiny particles like those in milk, or even molecules in the air (which happens to be why the sky is blue).
Whipped Marmite (and other foam) looks white because of what happens when light gets scattered not just once, but repeatedly, many times over. Since scattering occurs every time a particle of light travels into or out of an air bubble, the more bubbles there are, the more times it's going to be scattered. And even if it changes direction only slightly each time, the light won't go very far into the foam before its direction is essentially random, having entered and left all those different bubbles. Eventually, it will probably end up being thrown back out of the Marmite. On its journey, it will have passed through a lot of bubbles, but not actually that much of the light-absorbing Marmite. So there won't have been much opportunity for the brown goo to have absorbed the light, and what comes out is a mix of all the light that is thrown in. In daylight, that mix is white light, so the Marmite looks white.
Clouds, snow, sugar and milk
How about clouds? Well, it's the same 'multiple scattering' effect. Clouds contain small droplets of water which act a bit like the bubbles in foam. The droplets are much more spread out, so light can go many metres into a cloud without hitting one (scientists say it is much less 'optically thick' than whipped Marmite). However, once light does hit a drop it is scattered, and after hitting many droplets, and bouncing around inside the cloud for many hundreds of metres, the light will come back out again. In thin clouds, the light has a good chance of making it through all the way to the opposite side, which is why it's still light outside on an overcast day. However, in really thick clouds it's pretty unlikely that a particle of light will randomly make its way from the sunlit side, all the way through. So thick clouds look whiter on the top and ominously darker underneath.
It's not just clouds and foam. Multiple scattering also explains the colour of snow, powdered crystals like salt and sugar, and even milk. It doesn't really matter whether different wavelengths of light (eg red or blue light) are scattered differently – as they are with small particles such as those in milk – or scattered in the same way, as happens for bubbles and clouds. If there's a lot of scattering before much light is absorbed, most of what goes in will come back out again, and the substance will look white.
I'd like to thank Professor Craig F Bohren for his help with this article. Craig's book, Clouds in a Glass of Beer, also has one of the best explanations of multiple light scattering that I've found.
Brain Test Britain
Elsewhere on the BBC
Elsewhere on the web
Science speak
- Stephen Hawking, theoretical physicist (1942-present)
BBC iD
BBC iDBBC navigation
BBC links
BBC © 2014The BBC is not responsible for the content of external sites. Read more.
This page is best viewed in an up-to-date web browser with style sheets (CSS) enabled. While you will be able to view the content of this page in your current browser, you will not be able to get the full visual experience. Please consider upgrading your browser software or enabling style sheets (CSS) if you are able to do so.